The termination of persistent atrial fibrillation (AF) during catheter ablation has been associated in some, but not all, studies with reduced arrhythmia during clinical follow-up. We sought to determine the rate of persistent AF termination achievable with a stepwise ablation strategy, the predictors of AF termination, and the clinical outcomes associated with termination and nontermination. A total of 143 consecutive patients (age 62 ± 9 years, AF duration 5.7 ± 5.2 years) with persistent and longstanding persistent AF resistant to antiarrhythmic medication who presented in AF for catheter ablation were studied. Ablation was done with a stepwise approach, including pulmonary vein isolation, followed by complex fractionated atrial electrogram ablation and ablation of resultant atrial tachycardias. Clinical follow-up was then performed after a 2-month blanking period to assess arrhythmia recurrence, defined as AF or atrial tachycardia lasting ≥30 seconds. AF termination by ablation was achieved in 95 (66%) of the 143 patients. Multivariate predictors of AF termination included longer baseline AF cycle length (p <0.001) and smaller left atrial size (p = 0.002). AF termination by ablation was associated with both a lower incidence of arrhythmia recurrence after a single procedure without antiarrhythmic drugs (p = 0.01) and overall clinical success (single or multiple procedures, with or without antiarrhythmic drugs; p = 0.005). On multivariate analysis, the predictors of overall clinical success included AF termination by ablation (p = 0.001), a shorter ablation duration (p = 0.002), younger age (p = 0.02), male gender (p = 0.03), and the presence of hypertension (p = 0.03). In conclusion, among patients with persistent AF, termination of AF by ablation can be achieved in most patients and is associated with reduced recurrence of arrhythmia.
Catheter ablation of atrial fibrillation (AF) with the procedural end point of pulmonary vein isolation (PVI) is now a widely accepted procedure with generally good efficacy for patients with paroxysmal AF. However, PVI alone typically produces lower clinical success rates for ablation of persistent AF. To improve the efficacy of ablation of persistent AF, additional ablation strategies have been described, including targeting of complex fractionated atrial electrograms (CFAEs) (with or without PVI), linear ablation, and a stepwise approach generally involving PVI followed by CFAE and linear ablation, often with the goal of termination of AF by ablation. Additional ablation beyond PVI has been found to substantially increase the clinical success in regard to the freedom from recurrent AF but is associated with additional procedural risks, longer procedural and fluoroscopic times, and an increased risk of postablation atrial tachycardias (ATs), which can be highly symptomatic and often require repeat ablation procedures. Some, but not all, studies have suggested that termination of AF by ablation is associated with a lower risk of recurrent arrhythmia compared to failure to terminate AF with the need for electrical cardioversion. In the present analysis, we sought to determine the factors associated with termination of AF using a stepwise ablation approach and to determine whether AF termination and other baseline and procedural factors are associated with arrhythmia recurrence after ablation.
Methods
Our study population consisted of 143 consecutive patients who underwent a first catheter ablation of AF in the Electrophysiology Laboratory at the Massachusetts General Hospital (Boston, Massachusetts) from 2008 to 2010 for persistent and longstanding AF (as defined by the 2006 American College of Cardiology/American Heart Association/European Society of Cardiology consensus AF guidelines ). All patients had failed to maintain sinus rhythm despite cardioversion and treatment with ≥1 antiarrhythmic drug before ablation. Only patients who presented for the ablation procedure in AF were included. For patients taking antiarrhythmic drugs leading up to the procedure, these drugs were withheld for 4 to 5 half-lives before the procedure, except for amiodarone, which was typically held for 2 to 3 months before the ablation procedure. The demographic variables for the study patients are listed in Table 1 .
| Variable | All | AF Termination | p Value | |
|---|---|---|---|---|
| Yes | No | |||
| Patients (n) | 143 | 95 | 48 | |
| Women | 34 (20%) | 27 (30%) | 7 (10%) | 0.07 |
| Age (years) | 62 ± 9 | 63 ± 9 | 62 ± 8 | 0.60 |
| Left atrial size (mm) | 45 ± 7.7 | 44 ± 7 | 47 ± 8 | 0.03 |
| Left ventricular ejection fraction (%) | 57 ± 11.2 | 59 ± 11 | 55 ± 11 | 0.03 |
| Moderate or severe mitral regurgitation | 15% (21) | 12% (11) | 21% (10) | 0.14 |
| Coronary artery disease | 28 (20%) | 19 (20%) | 9 (19%) | 0.86 |
| Hypertension | 86 (60%) | 57 (60%) | 29 (60%) | 0.96 |
| Diabetes mellitus | 20 (14%) | 13 (14%) | 7 (15%) | 0.88 |
| Atrial fibrillation duration (years) | 5.7 ± 5.2 | 5.9 ± 5.2 | 5.3 ± 5.1 | 0.57 |
| Antiarrhythmic drug at discharge | 113 (79%) | 72 (76%) | 41 (85%) | 0.18 |
| Antiarrhythmic drug at follow-up | 38 (27%) | 22 (23%) | 16 (33%) | 0.19 |
| Ablation time (minutes) | 74 ± 20 | 75 ± 21 | 73 ± 18 | 0.61 |
| Procedure time (minutes) | 284 ± 71 | 284 ± 75 | 283 ± 65 | 0.91 |
| Baseline atrial fibrillation cycle length (ms) | 170 ± 24 | 175 ± 27 | 159 ± 13 | <0.001 |
Warfarin was continued throughout the ablation period, with a goal of an international normalized ratio of 2.0 to 3.5 on the day of the procedure. Transesophageal echocardiography to exclude thrombus in the left atrium and left atrial appendage was performed for patients with subtherapeutic international normalized ratio values during the month leading up to the ablation. The ablation procedures were performed with the patient under general anesthesia. Right and left femoral venous access was obtained. A diagnostic catheter was placed in the coronary sinus. Under fluoroscopic and intracardiac echocardiographic (Acu Nav Diagnostic Ultrasound Catheter, Siemens Medical Systems, Issaquah, Washington) guidance, 2 transseptal punctures were performed by way of the right femoral venous access, and 8F SL1 (Daig Division, St. Jude Medical, Minnetonka, Minnesota) and 8.5F Agilis (St. Jude Medical) sheaths were advanced into the left atrium. Bipolar electrograms were recorded at a bandpass of 30 to 500 Hz (Prucka Cardio Lab IT System, GE Healthcare, Milwaukee, Wisconsin). Three-dimensional mapping of the left atrium was performed using the CARTO-XP and CARTO–3 systems (Biosense Webster, Diamond Bar, California) or the EnSite System (St. Jude Medical) and the use of multi-electrode mapping catheters. The baseline AF cycle length was recorded in the left atrial appendage before ablation by averaging 10 cycles, as previously described.
Ablation was performed using a stepwise approach. Isolation of all pulmonary veins using wide area antral ablation and verification of entrance block during AF using the multi-electrode mapping catheter was performed as a first step in all patients. No patients in the present study were converted to sinus rhythm during ablation before isolation of the pulmonary veins. If AF persisted after PVI, CFAEs were then identified and ablated in the left atrium and coronary sinus, with CFAEs verified by either visual assessment or automated algorithms (Biosense Webster and St. Jude Medical). When CFAEs were encountered in the posterior left atrium, this area was isolated. If AF persisted after all detectable CFAEs were ablated in the left atrium and coronary sinus, mapping and ablation of CFAEs in the right atrium was performed ( Figure 1 ). If AF persisted after PVI and ablation of all detectable CFAE regions in the left atrium and right atrium, electrical cardioversion was performed. If the AF converted during ablation to AT, activation and entrainment mapping of the AT followed by ablation targeting the critical isthmus (i.e., mitral isthmus, cavotricuspid isthmus, left atrial roof) for macro-reentrant AT or the area of earliest activation for focal AT was performed ( Figure 2 ). When multiple ATs were encountered, these were targeted sequentially by ablation, with the goal of achieving sinus rhythm by ablation alone. When patients were converted to sinus rhythm by ablation (either directly from AF to sinus or from AF to AT to sinus rhythm), attempts were not made to reinduce AF. Once sinus rhythm was achieved, verification of the entrance and exit block of all pulmonary veins and block along all linear ablations was performed.
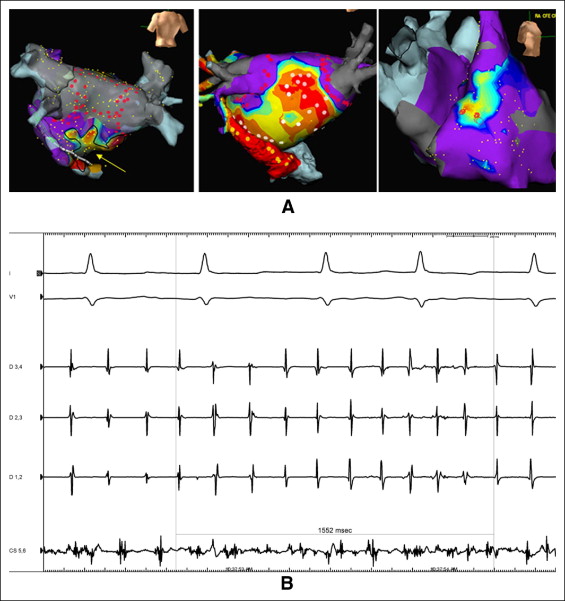
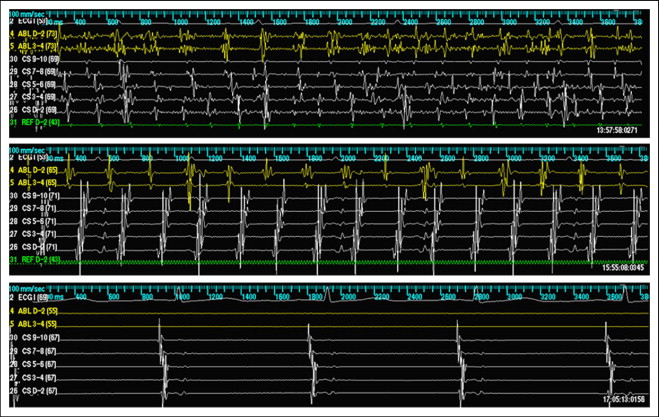
The patients were followed up in the office at regularly scheduled visits, generally 4 to 6 weeks after ablation and 3, 6, and 12 months after ablation, and yearly thereafter, with additional visits scheduled if symptoms suggestive of arrhythmia recurrence were noted. Recurrence of arrhythmia (symptomatic or asymptomatic) was determined from electrocardiograms performed during routine office visits (and at urgent visits for symptomatic arrhythmias) and outpatient Holter monitoring or multiweek event monitoring using event monitors with auto-triggering for AF, AT, and other atrial arrhythmias (CardioNet, Conshohocken, Pennsylvania; and LifeWatch, Rosemont, Illinois). Antiarrhythmic drugs were continued at the discretion of the operator for 1 to 2 months after the ablation procedure. The antiarrhythmic drugs were then discontinued for patients free of arrhythmia after this period. The first 2 months after the ablation was considered a blanking period, during which recurrent arrhythmias were not considered procedural failure. After the 2-month blanking period, any asymptomatic or symptomatic atrial tachyarrhythmia lasting ≥30 seconds (AF or AT, including typical and atypical forms of atrial flutter) were recorded as procedural failures. An assessment was also made regarding whether patients were taking antiarrhythmic drugs at discharge after the ablation procedure, at the follow-up visits, and at the detection of postablation arrhythmias. Patients who underwent repeat ablation procedures were followed up in the same fashion for additional recurrences. Overall clinical success was defined as freedom from recurrent arrhythmias (after the 2-month blanking period) after the last ablation procedure had been performed.
Patients with recurrent arrhythmias after the blanking period were offered repeat ablation procedures. Repeat procedures were performed, targeting the documented recurrent arrhythmia. In all cases, electrical isolation of all pulmonary veins and block along all ablation lines from the initial procedure was assessed, and additional ablation was performed until all pulmonary veins were reisolated and all previous ablation lines demonstrated block. In cases of recurrent AF (assuming persistence of AF after these maneuvers), CFAE mapping and ablation was performed as described for the initial procedure. When AT was the mode of recurrence (or occurred after the described steps), AT was targeted as described previously. Additional clinical follow-up was then performed, as described previously, to assess for recurrent arrhythmia after the repeat procedure.
The data are reported as the mean ± SD. Comparisons between groups were performed with a 2-tailed unpaired Student’s t test for continuous variables and Fisher’s exact test for categorical variables. Freedom from arrhythmia was assessed from the end of the 2-month blanking period after ablation using Kaplan-Meier analysis. Multivariate analysis to seek the predictors of freedom from AF was performed using a Cox proportional hazards model. The estimate of predictors of AF was obtained by logistic regression analysis. The statistical package Stata (StataCorp, College Station, Texas) was used in these analyses. A p value of ≤0.05 was considered statistically significant for all analyses.
Results
A total of 143 patients were included in the present analysis. Of these 143 patients, ablation terminated AF in 95 (66%). In 48 (34%), AF did not terminate with ablation, and they required cardioversion at the end of the procedure. Of the 95 patients whose AF terminated with ablation, 24 (25%) terminated directly from AF to sinus rhythm, and 71 (75%) converted from AF to AT with ablation, and the AT was then ablated to sinus rhythm. The significant univariate predictors of termination of AF by ablation included longer baseline AF cycle length, smaller left atrial size, and greater left ventricular ejection fraction ( Table 1 ). On multivariate analysis, the predictors of AF termination by ablation during the index procedure included longer baseline AF cycle length (odds ratio 1.06, p <0.001) and smaller left atrial size (odds ratio 0.92, p = 0.002). A total of 180 ablation procedures were performed in these 143 patients (average 1.3 procedures/patient).
Major complications occurred in 3 (1.7%) of the 180 procedures, including pleuro-pericarditis requiring chest tube drainage of pleural fluid in 1 patient and major femoral groin access site complications requiring blood transfusion in 1 patient and surgical repair in 1 patient. No strokes or transient ischemic attacks, atrial-esophageal fistula, cardiac tamponade, or procedure-related deaths occurred. All patients recovered completely from these complications.
The patients were followed up for recurrent atrial arrhythmias after the 2-month blanking period after ablation. A Kaplan-Meier plot for overall clinical success (freedom from AF/AT after single or multiple procedures with or without antiarrhythmic drugs) for all 143 patients is shown in Figure 3 . Recurrent arrhythmia after the 2-month blanking period was noted in 46 (48%) of the 95 patients who had termination of AF by ablation and 28 (58%) of the 48 patients without termination of AF by ablation. At redo ablation, 1 patient in each group (with or without termination by ablation) had persistent isolation of all pulmonary veins (p = NS). Of the patients with recurrence of arrhythmia after the initial procedure, the incidence recurrence in the form of AT was greater in the 95 patients with termination of AF by ablation (26 of 46 [57%] with AT). Those without AF termination by ablation had a greater incidence of recurrence in the form of AF (13 [46%] of 28 with AF), but this difference was not statistically significant by Fisher’s exact test (p = 0.48).




