Patients with chronic kidney disease have a worse cardiovascular prognosis than those without. The aim of this study was to determine the incremental prognostic value of coronary computed tomographic angiography in predicting mortality across the entire spectrum of renal function in patients with known or suspected coronary artery disease (CAD). A large international multicenter registry was queried, and patients with left ventricular ejection fraction (LVEF) and creatinine data were screened. National Cholesterol Education Program Adult Treatment Panel III risk was calculated. Coronary computed tomographic angiographic results were evaluated for CAD severity (normal, nonobstructive, or obstructive) and an LVEF <50%. Patients were followed for the end point of all-cause mortality. Among 5,655 patients meeting the study criteria, follow-up was available for 5,572 (98.9%; median follow-up duration 18.6 months). All-cause mortality (66 deaths) significantly increased with every 10-unit decrease in renal function (hazard ratio [HR] 1.23, 95% confidence interval [CI] 1.07 to 1.41). All-cause mortality occurred in 0.33% of patients without coronary atherosclerosis, 1.82% of patients with nonobstructive CAD, and 2.43% of patients with obstructive CAD. Multivariate Cox proportional-hazards models revealed that impaired renal function (HR 2.29, 95% CI 1.65 to 3.18), CAD severity (HR 1.81, 95% CI 1.31 to 2.51), and an abnormal LVEF (HR 4.16, 95% CI 2.45 to 7.08) were independent predictors of all-cause mortality. In conclusion, coronary computed tomographic angiographic measures of CAD severity and the LVEF provide effective risk stratification across a wide spectrum of renal function. Furthermore, renal dysfunction, CAD severity, and the LVEF have additive value for predicting all-cause death in patients with suspected obstructive CAD.
Previous single-center and multicenter trials have established the utility of coronary computed tomographic angiography (CCTA) for the diagnosis, management, and prognosis of patients with established or suspected coronary artery disease (CAD). Unless receiving renal replacement therapy, patients with end-stage renal failure are not considered ideal candidates for CCTA because of the risk for contrast agent–induced nephrotoxicity. However, emerging prospective clinical investigations from high-risk patient populations receiving intravenous contrast media for computed tomography suggest that the incidence and serious negative clinical outcomes are much less common than previously believed. Moreover, the risk for nephrotoxicity can be reduced with proper patient preparation. Globally, a significant number of patients with varying degree of renal impairment undergo CCTA as part of their cardiac risk evaluation. Despite the significant numbers and obvious clinical implications, to the best of our knowledge, the prognostic value of CCTA across the different stages of renal impairment has not been evaluated in a large-scale multicenter trial. The main aim of this study was to determine the incremental prognostic value of CCTA in predicting mortality across the entire spectrum of renal function in a large prospective cohort of patients with known or suspected CAD recruited in Coronary Computed Tomography Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry (CONFIRM).
Methods
Details of CONFIRM have been described previously. Briefly, centers with 64-slice computed tomographic scanners participating in this registry prospectively contributed to coronary computed tomographic angiographic databases as part of a large multicenter observational registry. Qualifying sites contributed baseline demographics, cardiac risk factors, drug history, findings on CCTA, and outcomes. From February 2003 to December 2009, 27,125 consecutive patients underwent CCTA at 12 enrolling centers in 6 countries (Canada, Germany, Italy, Korea, Switzerland, and the United States) and were prospectively entered. Each enrolling center contributed 499 to 4,912 patients for analysis. For the present study, patients with left ventricular ejection fraction (LVEF) and serum creatinine assessments were screened for inclusion. Patients with histories of coronary revascularization (coronary artery bypass grafting and/or percutaneous coronary intervention), congenital heart disease, or cardiac transplantation were excluded from the present investigation. Follow-up procedures were approved by the institutional review board at each site.
Medical histories were obtained for all patients. Patients’ pretest probability for obstructive CAD was calculated using age, gender, and symptoms. Each patient’s risk for future cardiac events was assessed using National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III guidelines.
The coronary computed tomographic angiographic protocol, data acquisition, image postprocessing, and interpretation for the present study cohort were performed in accordance with the guidelines of the Society of Cardiovascular Computed Tomography.
Coronary diameter stenoses were graded using a 3-point scale (normal, nonobstructive [<50%], or obstructive [≥50%]). Abnormal LVEFs were calculated using end-diastolic and end-systolic volumes, and an LVEF <50% was considered abnormal.
Serum creatinine was used to assess renal function in all study participants. Estimated glomerular filtration rate (eGFR) was calculated for each patient using the Levey Modification of Diet in Renal Disease (MDRD) formula (eGFR = 175 × standardized serum creatinine − 1.154 × age − 0.203 × 0.742 [if female]). The eGFR values were categorized as <30, 30 to 59, 60 to 89, or ≥90 ml/min/1.73 m 2 , on the basis of the Kidney Disease Outcomes Quality Initiative classification of renal function.
Patient follow-up for all-cause mortality was performed by each local institution by telephone interview, with validation of reported death through medical records whenever possible and/or a national death registry.
Statistical analysis was performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina), and statistical significance was defined as p <0.05. Categorical data are presented as percentage frequencies. Continuous variables are expressed as mean ± SD. To compare patient characteristics, Wilcoxon’s rank-sum test was used to compare continuous variables and the chi-square test was used for categorical variables. Prognostic values of eGFR, CAD severity, and the LVEF were assessed for univariate and multivariate associations with all-cause mortality using Cox proportional-hazard models. For risk-adjusted analysis, the independent prognostic value of eGFR, CAD severity, and the LVEF was assessed by controlling for clinical predictors (NCEP ATP III score) and creating adjusted survival curves. Model overfitting was considered, and the proportional-hazards assumption was met. The incremental value of eGFR, CAD severity, and the LVEF was calculated by defining the clinical predictor model, followed by the addition of eGFR, CAD severity, and the LVEF. Receiver-operator characteristic curves were constructed for models of clinical predictors only, clinical predictors plus eGFR, clinical predictors plus eGFR plus CAD severity, and clinical predictors plus eGFR plus CAD severity plus the LVEF. Areas under the receiver-operating characteristic curves (with 95% confidence intervals [CIs]) were compared to evaluate the discriminative ability of eGFR over clinical predictors, CAD severity over clinical predictors plus eGFR, and the LVEF over clinical predictors plus eGFR plus CAD severity to predict all-cause mortality.
Results
In the whole registry, 27,125 patients were screened with CCTA at 12 participating centers. Of the 5,864 patients with clinical variables, creatinine value, CAD severity, and LVEF assessments (normal vs abnormal LVEFs), 209 patients were excluded because of histories of coronary revascularization, congenital heart disease, or cardiac transplantation. The final study population included 5,655 patients. Follow-up was available for 5,572 (98.9%; mean age 55.4 ± 12.7 years, 52% men), with a median follow-up duration of 18.6 months (range 13.8 to 24.2). Table 1 lists the baseline characteristics of the study cohort. Patients with impaired renal function were older and had a higher prevalence of hypertension, diabetes, and dyslipidemia. Higher incidence of chest pain and dyspnea as indications for CCTA were observed in patients with impaired renal function. Absolute LVEF measurements were available in 52% of the analyzed population. The mean LVEF when assessed as a continuous variable was 63.2 ± 11.4%, with significantly lower values in patients with impaired renal function.
| Variable | All Patients (n = 5,572) | eGFR (ml/min/1.73 m 2 ) | p Value | |||
|---|---|---|---|---|---|---|
| ≥90 (n = 2,130) | 60–89 (n = 2,827) | 30–59 (n = 580) | <30 (n = 35) | |||
| Age (yrs) | 55.4 ± 12.7 | 50.1 ± 11.7 | 57.0 ± 11.7 | 66.5 ± 11.3 | 59.3 ± 13.5 | <0.001 |
| Men | 2,881 (51.7%) | 1,143 (53.7%) | 1,530 (54.1%) | 190 (32.8%) | 18 (51.4%) | <0.001 |
| Body mass index (kg/m 2 ) | 28.7 ± 5.9 | 28.6 ± 6.0 | 28.7 ± 5.7 | 29.0 ± 6.5 | 28.3 ± 6.6 | 0.358 |
| Creatinine (mg/dl) | 0.9 ± 0.6 | 0.7 ± 0.1 | 1.0 ± 0.2 | 1.2 ± 0.2 | 6.5 ± 3.2 | <0.001 |
| Smoker/ex-smoker | 2,181 (39.1%) | 833 (39.1%) | 1,107 (39.2%) | 229 (39.5%) | 12 (34.3%) | 0.945 |
| Hypertension | 2,748 (49.3%) | 827 (38.8%) | 1,468 (51.9%) | 422 (72.8%) | 31 (88.6%) | <0.001 |
| Dyslipidemia | 2,783 (49.9%) | 887 (41.6%) | 1,499 (53.0%) | 376 (64.8%) | 21 (60.0%) | <0.001 |
| Diabetes | 692 (12.4%) | 228 (10.7%) | 332 (11.7%) | 113 (19.5%) | 19 (54.3%) | <0.001 |
| Family history of CAD | 2,204 (39.6%) | 847 (39.8%) | 1,149 (40.6%) | 198 (34.1%) | 10 (28.6%) | 0.016 |
| Indication for study | ||||||
| Chest pain | 3,635 (65.2%) | 1,520 (71.4%) | 1,745 (61.7%) | 352 (60.7%) | 18 (51.4%) | <0.001 |
| Nonanginal chest pain | 2,396 (43.0%) | 1,199 (56.3%) | 1,026 (36.3%) | 166 (28.6%) | 5 (14.3%) | <0.001 |
| Atypical angina pectoris | 776 (13.9%) | 217 (10.2%) | 445 (15.7%) | 107 (18.4%) | 7 (20.0%) | <0.001 |
| Typical angina pectoris | 463 (8.3%) | 104 (4.9%) | 274 (9.7%) | 79 (13.6%) | 6 (17.1%) | <0.001 |
| Dyspnea | 584 (10.5%) | 148 (6.9%) | 329 (11.6%) | 100 (17.2%) | 7 (20.0%) | <0.001 |
| Coronary artery narrowing | ||||||
| None | 2,752 (49.4%) | 1,333 (62.6%) | 1,231 (43.5%) | 179 (30.9%) | 9 (25.7%) | <0.001 |
| Nonobstructive | 1,872 (33.6%) | 555 (26.0%) | 1,044 (36.9%) | 260 (44.8%) | 13 (37.1%) | <0.001 |
| Obstructive | 948 (17.0%) | 242 (11.4%) | 552 (19.5%) | 141 (24.3%) | 13 (37.1%) | <0.001 |
| Abnormal LVEF (<50%) | 413 (7.4%) | 94 (4.4%) | 234 (8.3%) | 77 (13.3%) | 8 (22.9%) | <0.001 |
| LVEF (%) ∗ | 63.2 ± 11.4 | 62.21 ± 10.5 | 63.4 ± 10.9 | 64.0 ± 13.9 | 60.8 ± 15.3 | 0.026 |
∗ Analysis of the LVEF was performed in 2,896 patients (51.97%).
In total, 2,820 patients (51.6%) had abnormal results on CCTA, with 948 patients (17%) demonstrating obstructive CAD. The prevalence of abnormal results on CCTA increased with worsening renal function (37.4% and 74.2% in patients with eGFRs ≥90 vs <30 ml/min/1.73 m 2 , respectively; Table 1 ).
At follow-up, all-cause mortality was observed in 66 patients (1.18%), with an annualized mortality rate for the entire study cohort of 0.62%. All-cause mortality significantly increased with every 10-unit decrease in eGFR (hazard ratio [HR] 1.23, 95% CI 1.07 to 1.41). An eGFR ≥90 ml/min/1.73 m 2 conferred excellent survival, with only 16 of the total 2,130 patients (0.75%) dying in this group ( Table 2 ). Decrease in eGFR was associated with a significant increase (HR 2.97, 95% CI 2.13 to 4.13) in all-cause mortality, with 0.75%, 0.81%, 3.45%, and 20% patients dying during study follow-up for eGFRs ≥90, 60 to 89, 30 to 59, and <30 ml/min/1.73 m 2 , respectively ( Table 3 ).
| Variable | Death | HR (95% CI) | p Value | |
|---|---|---|---|---|
| No (n = 5,506) | Yes (n = 66) | |||
| Age (yrs) | 55.3 ± 12.6 | 64.7 ± 14.7 | 1.06 (1.04–1.09) | <0.001 |
| Men | 2,847 (51.7%) | 34 (51.5%) | 1.01 (0.62–1.63) | 0.984 |
| Body mass index (kg/m 2 ) | 28.7 ± 5.9 | 26.9 ± 6.6 | 0.94 (0.89–0.99) | 0.014 |
| Cardiac risk factors | ||||
| Diabetes | 671 (12.2%) | 21 (31.8%) | 3.33 (1.98–5.58) | <0.001 |
| Dyslipidemia | 2,757 (50.1%) | 26 (39.4%) | 0.63 (0.38–1.03) | 0.063 |
| Hypertension | 2,695 (48.9%) | 53 (80.3%) | 4.26 (2.32–7.81) | 0.001 |
| Family history of CAD | 2,183 (39.6%) | 21 (31.8%) | 0.73 (0.43–1.22) | 0.232 |
| Smoker/ex-smoker | 2,151 (39.1%) | 30 (45.5%) | 1.38 (0.85–2.25) | 0.189 |
| eGFR (ml/min/1.73 m 2 ) | 2.97 (2.13–4.13) | <0.001 | ||
| ≥90 | 2,114 (38.4%) | 16 (24.2%) | ||
| 60–89 | 2,804 (50.9%) | 23 (34.8%) | ||
| 30–59 | 560 (10.2%) | 20 (30.3%) | ||
| <30 | 28 (0.5%) | 7 (10.6%) | ||
| eGFR (10-unit decrease) | 78.3 ± 20.3 | 70.0 ± 38.1 | 1.23 (1.07–1.41) | 0.003 |
| NCEP ATP III score | 2.88 (1.97–4.22) | <0.001 | ||
| Low risk | 1,462 (26.6%) | 8 (12.1%) | ||
| Intermediate risk | 3,084 (56.0%) | 27 (40.9%) | ||
| High risk | 960 (17.4%) | 31 (47.0%) | ||
| Coronary artery narrowing | 2.40 (1.77–3.27) | <0.001 | ||
| None | 2,743 (49.8%) | 9 (13.6%) | ||
| <50% | 1,838 (33.4%) | 34 (51.5%) | ||
| ≥50% | 925 (16.8%) | 23 (34.8%) | ||
| Abnormal LVEF (<50%) | 391 (7.1%) | 22 (33.3%) | 6.64 (3.98–11.10) | <0.001 |
| LVEF (10% decrease) ∗ | 63.3 ± 11.2 | 55.3 ± 18.6 | 1.54 (1.31–1.82) | <0.001 |
∗ Analysis of the LVEF was performed in 2,896 patients (51.97%).
| eGFR (ml/min/1.73 m 2 ) | n | All Cause Death |
|---|---|---|
| All patients | 5,572 | 66 (1.18%) |
| ≥90 | 2,130 | 16 (0.75%) |
| 60–89 | 2,827 | 23 (0.81%) |
| 30–59 | 580 | 20 (3.45%) |
| <30 | 35 | 7 (20.0%) |
The absence of coronary atherosclerosis conferred an excellent prognosis, with only 9 deaths (0.32%) in this group. Patients with nonobstructive and obstructive CAD had all-cause death rates of 1.82% and 2.43%, respectively. An abnormal LVEF (HR 6.64, 95% CI 3.98 to 11.10) and a 10% decrease in the LVEF (HR 1.54, 95% CI 1.31 to 1.82) were associated with significant increase in all-cause mortality. A significant increase in all-cause mortality was also seen with progressive decrease in eGFR and increase in CAD severity ( Figure 1 ).
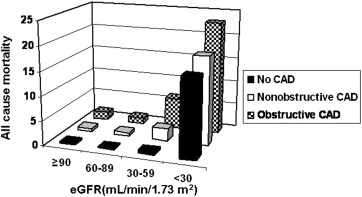
In univariate analysis, clinical parameters (age, body mass index, diabetes, hypertension, and NCEP ATP III score), eGFR, and coronary computed tomographic angiographic parameters (severity of CAD and an abnormal LVEF) were significant predictors of all-cause mortality ( Tables 2 and 4 ). For the risk-adjusted analysis, the NCEP ATP III score was used as the clinical predictor to determine the incremental value of eGFR and coronary computed tomographic angiographic measures because it combines age, gender, and cardiac factors into a single measure. A multivariate Cox model of eGFR, CAD severity, and an abnormal LVEF was tested. The eGFR, CAD severity, and an abnormal LVEF remained independent predictors of all-cause mortality after adjusting for the clinical predictor ( Figures 2 and 3 , Table 4 ). A significant increase (p <0.001) in the global chi-square value for the Cox model was observed after the addition of eGFR (global chi-square = 50.64) to the clinical predictor (global chi-square = 29.56). Additions of CAD severity and an abnormal LVEF to models with clinical predictor plus eGFR (global chi-square = 61.85) and clinical predictor plus eGFR plus CAD severity (global chi-square = 98.78) as variables, respectively, also demonstrated significant increases in p values (<0.001).
| Model | HR (95% CI) | p Value |
|---|---|---|
| Clinical variables | ||
| NCEP ATP III score | 2.88 (1.97–4.22) | <0.001 |
| Clinical variables plus eGFR | ||
| NCEP ATP III score | 2.42 (1.64–3.57) | <0.001 |
| eGFR (ml/min/1.73 m 2 ) | 2.48 (1.79–3.43) | <0.001 |
| ≥90 | 1.0 | — |
| 60–89 | 0.94 (0.50–1.79) | 0.857 |
| 30–59 | 3.48 (1.79–6.78) | <0.001 |
| <30 | 17.72 (7.05–44.56) | <0.001 |
| Clinical variables plus eGFR plus CAD severity | ||
| NCEP ATP III score | 2.08 (1.38–3.12) | <0.001 |
| eGFR (ml/min/1.73 m 2 ) | 2.29 (1.65–3.18) | <0.001 |
| ≥90 | 1.0 | — |
| 60–89 | 0.82 (0.43–1.56) | 0.543 |
| 30–59 | 2.91 (1.49–5.69) | 0.002 |
| <30 | 13.16 (5.16–33.56) | <0.001 |
| Coronary narrowing | 1.81 (1.31–2.50) | <0.001 |
| None | 1.0 | — |
| <50% | 4.19 (1.98–8.83) | <0.001 |
| ≥50% | 4.28 (1.94–9.47) | <0.001 |
| Clinical variables plus eGFR plus CAD severity plus abnormal LVEF | ||
| NCEP ATP III score | 1.86 (1.24–2.79) | 0.003 |
| eGFR (ml/min/1.73 m 2 ) | 2.01 (1.44–2.81) | <0.001 |
| ≥90 | 1.0 | — |
| 60–89 | 0.74 (0.39–1.41) | 0.361 |
| 30–59 | 2.33 (1.18–4.61) | 0.015 |
| <30 | 8.74 (3.32–23.05) | <0.001 |
| Coronary narrowing | 1.81 (1.31–2.51) | <0.001 |
| None | 1.0 | — |
| <50% | 4.02 (1.91–8.48) | <0.001 |
| ≥50% | 4.24 (1.91–9.37) | <0.001 |
| Abnormal LVEF (<50%) | 4.16 (2.45–7.08) | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


