The aim of this study was to evaluate platelet reactivity and 30-day bleeding events in patients treated with prasugrel 10 mg after acute coronary syndromes. A total of 444 patients with acute coronary syndromes treated with percutaneous coronary intervention and prasugrel 10 mg/day were monitored by measurement of the vasodilator-stimulated phosphoprotein (VASP) index 2 to 4 weeks after hospital discharge. Platelet reactivity was also assessed using the VerifyNow P2Y 12 assay and light transmission aggregometry. Bleeding events (per the Bleeding Academic Research Consortium [BARC] definition) and ischemic events (death, myocardial infarction, and definite stent thrombosis) were collected over 30 days of follow-up. Two thirds of the patients presented with ST-segment elevation myocardial infarctions, 28.8% had diabetes, and 12.4% were aged >75 years. High on-treatment platelet reactivity according to 3 prespecified definitions (VASP index ≥50%, platelet reactivity ≥235 P2Y 12 reaction units, and residual platelet reactivity ≥46.2%) was found in 6.8%, 3.4%, and 3.2% of patients, respectively. Obesity (body mass index >30 kg/m 2 ) and multivessel disease were the only independent factors associated with high on-treatment platelet reactivity (p = 0.006 and p = 0.045, respectively). At 30 days, there was no major bleeding complication (BARC grade 3 or 5), and 1.6% of patients had recurrent ischemic events. Nuisance bleeding (BARC grade 1) and minor bleeding (BARC grade 2) occurred in 14.2% (n = 63) and 2.5% (n = 11) of patients, respectively, but were not predicted by VASP index. In conclusion, patients with acute coronary syndromes receiving maintenance doses of prasugrel have low rates of HPR and ischemic events within the first month. Minor or minimal bleeding is frequent, but not major bleeding. VASP was poorly correlated with the risk for minor or minimal bleeding.
Prasugrel, a third-generation thienopyridine, induces more rapid, more potent, and more predictable platelet inhibition than clopidogrel. It was associated with a greater reduction of ischemic events with an excess of bleeding events in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis In Myocardial Infarction 38 (TRITON–TIMI 38) trial.
Limited information is available on the level of platelet inhibition obtained in the TRITON–TIMI 38 study patients and even less in the real world population. Recent data suggest that high on-treatment platelet reactivity (HPR) is present in 1/4 of patients after prasugrel loading, with a possible relation to ischemic events. The rate of HPR in patients with acute coronary syndromes (ACS) receiving 10-mg maintenance doses of prasugrel has not been well explored. Moreover, the relation of platelet reactivity to safety, which remains the main concern for chronic treatment, is not known. Therefore, we decided to routinely evaluate platelet reactivity on prasugrel 10-mg maintenance dose and its relation to clinical tolerance and outcomes.
Methods
From February 2010 to August 2011, all consecutive patients with ACS managed invasively with percutaneous coronary intervention at 2 high-volume tertiary centers (Pitié-Salpêtrière University Hospital, Paris, France, and La Timone University Hospital, Marseille, France) and discharged on a maintenance dose of prasugrel 10 mg (the only dose available in France) were invited to participate in the study. To be eligible for this study, patients had to have stents implanted and be treated with the combination of low-dose aspirin (75 to 250 mg/day) and prasugrel 10 mg/day. Patients with histories of stroke were excluded. The decision for treatment with prasugrel 10-mg maintenance dose was left to the discretion of the treating physician according to the expected risk and benefit in each patient.
Patients were included on the day of discharge. All patients were scheduled for visits 2 to 4 weeks after discharge to check compliance with treatment and measure platelet reactivity. Blood samples were collected by venipuncture into 3.2% citrate Vacuette tubes (Becton, Dickinson, Franklin Lakes, New Jersey) after having discarded the first 2 to 4 ml of blood to avoid spontaneous platelet activation. Samples were processed <2 hours after blood drawing for platelet function testing. Clinical follow-up was performed at 1-month follow-up. The institutional review board of Pitié-Salpêtrière Hospital approved this study.
The phosphorylation of the vasodilator-stimulated phosphoprotein (VASP) was measured using a Beckman Coulter FC500 cytometer (Beckman Coulter, Villepinte, France) using Platelet VASP kits (Diagnostica Stago [Biocytex], Asnières, France) according to the manufacturer’s instructions and as previously described. Briefly, blood samples were incubated in vitro with adenosine diphosphate (ADP) and/or prostaglandin E 1 (PGE 1 ) before fixation. The VASP platelet reactivity index (PRI) was calculated from the mean fluorescence intensity of each condition as follows: VASP PRI = [(mean fluorescence intensity of PGE 1 ) − (mean fluorescence intensity of PGE 1 + ADP)/mean fluorescence intensity of PGE 1 ] × 100.
Platelet-rich plasma was obtained by centrifugation of citrated whole blood at 100g for 10 minutes at room temperature. Platelet poor plasma was obtained by further centrifugation at 4,500g for 15 minutes. In vitro platelet aggregation in platelet-rich plasma was measured at 37°C in a light transmission aggregometer (model 490-4D; Chrono-Log Corporation, Kordia, The Netherlands) and was induced by the addition of ADP (Sigma-Aldrich, Saint Quentin Fallavier, France) at a final concentration of 20 μmol/L. Residual platelet aggregation measured 6 minutes after the induction of aggregation by agonist was recorded. Prespecified criteria used to define nonevaluable samples were lack of sufficient signal, hemolysis, platelet-rich plasma platelet count <150,000/ml, and an unstable baseline.
Measurement of platelet response to prasugrel using the VerifyNow P2Y 12 assay (Accumetrics Corporation, San Diego, California) was done according to the package insert. Results are expressed in P2Y 12 reaction units (PRU) in response to isothrombin receptor–activating peptide and in response to ADP-PGE 1 . Isothrombin receptor–activating peptide strongly activates platelets despite of P2Y 12 receptor blockage by thienopyridine or aspirin and reflects platelet reactivity without treatment. The device provides an estimated inhibition (as a percentage) without prethienopyridine sample by reporting the ratio of the results of the ADP-PGE 1 and isothrombin receptor–activating peptide channels. Platelet aggregation and VerifyNow were available at 1 center, whereas VASP measurement was performed at both centers using the same method.
Our main objectives were (1) to evaluate the rate of HPR on prasugrel 10-mg maintenance dose using VASP PRI value ≥50% and VASP PRI value ≥60% and identify the independent correlates of HPR; (2) to confirm the rate of HPR in a subgroup of this population using 2 additional methods: light transmission aggregometry with residual platelet aggregation value ≥46.2% and VerifyNow P2Y 12 assay with PRU ADP-PGE 1 value ≥235 ; and (3) to evaluate the rate of bleeding events using the Bleeding Academic Research Consortium (BARC) definitions and examine the relation to platelet reactivity. Independent correlates of bleeding events were sought.
Cardiovascular ischemic events including death, recurrent myocardial infarction, and definite stent thrombosis (per the Academic Research Consortium definition) were also collected at 1-month follow-up.
Continuous variables are expressed as means ± SD and categorical data as percentages. Chi-square tests or Fisher’s exact tests were used for qualitative variables, and Student’s unpaired t test was used for continuous variables. Multivariate regression analyses were performed to identify independent correlates of HPR and bleeding events. After univariate analysis, all variables with p values <0.20 were introduced in the multivariate analysis. The variables entered in the HPR multivariate model were β-blocker use, body mass index >30 kg/m 2 , and 3-vessel disease. The variables entered in the bleeding multivariate model were VASP index <15.05%, age ≥75 years, gender, low weight (<60 kg), diabetes, 3-vessel disease, and creatinine clearance <60 ml/min. A backward procedure was used in the final model. Correlations between tests were assessed using Pearson’s test or Spearman’s test (when the distribution was not normal). Agreement among the 3 tests was calculated using the κ statistic. All p values are 2 sided, and p values <0.05 were considered significant. Analyses were performed using R version 12.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics and procedural details of the study population are listed in Table 1 . The mean age of the population was 60.1 ± 12 years, and 2/3 of the patients had ST-segment elevation myocardial infarctions, 28.8% had diabetes, and 12.4% were aged ≥75 years. Percutaneous coronary intervention was performed using a radial approach in 96.4% of patients. Complete 1-month follow-up was achieved in all patients. VASP measurement was available in all patients (n = 444), whereas VerifyNow P2Y 12 and platelet measurement by light transmission aggregometry were available in subgroups of the population of 52% (n = 232) and 49% (n = 219), respectively.
| Variable | Value |
|---|---|
| Age (yrs) | 60.1 ± 12 |
| Age ≥ 75 yrs | 55(12.4%) |
| Women | 66 (14.8%) |
| Weight (kg) | 79 ± 16 |
| Body mass index (kg/m 2 ) | 29.9 ± 5 |
| Low weight (<60 kg) | 32 (7.2%) |
| Diabetes | 128 (28.8%) |
| Hypercholesterolemia ∗ | 205 (46.2%) |
| Smoker | 209 (47%) |
| Hypertension † | 194 (43.7%) |
| Family history of coronary artery disease ‡ | 75 (16.9%) |
| ST-segment elevation myocardial infarction | 257 (57.8%) |
| Non–ST-segment elevation myocardial infarction/unstable angina | 163 (33.7%) |
| Stable angina | 22 (5%) |
| 1-vessel disease | 310 (69.9%) |
| 2-vessel disease | 89 (20%) |
| 3-vessel disease | 45 (10.1%) |
| Radial access | 428 (96.4%) |
| Drug-eluting stent | 271 (61%) |
| Platelet count (×10 3 /μl) | 254.1 ± 70 |
| Hemoglobin (g/dl) | 14.1 ± 1 |
| Creatinine clearance (ml/min) | 100 ± 73 |
| Creatinine clearance <60 ml/min | 62 (14%) |
| Creatinine clearance <30 ml/min | 11 (2.5%) |
| Left ventricular ejection fraction (%) | 53 ± 8 |
| Aspirin dose (mg) | 76 ± 10 |
| β blockers | 354 (79.7%) |
| Angiotensin-converting enzyme inhibitors | 349 (78.6%) |
| Calcium antagonists | 47 (10.6%) |
| Statins | 424 (95.5%) |
| Proton pump inhibitors | 378 (85.1%) |
∗ Fasting low-density lipoprotein cholesterol ≥131 mg/dl or receiving statin therapy at the time of inclusion.
† Systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg.
‡ One or more first-degree relatives developed coronary artery disease at <55 yrs of age (men) or <65 yrs of age (women).
HPR was observed in 6.7% (30 of 444) with the VASP PRI on flow cytometry. Using a cut point of 60% PRI, the rate of HPR was 3.2% (14 of 444). This incidence was confirmed with the other 2 methods: 3.4% (8 of 232) with the VerifyNow P2Y 12 assay and 3.2% (7 of 219) with light transmission aggregometry, according to each prespecified definition (see Figure 1 ). No problems of compliance with prasugrel were detected in this population. The correlations between the different tests were as follows: residual platelet aggregation and PRU, r = 0.66 (95% confidence interval [CI] 0.57 to 0.73, p <0.0001); VASP PRI and PRU, r = 0.61 (95% CI 0.57 to 0.72, p <0.0001); and VASP PRI and residual platelet aggregation, r = 0.42 (95% CI 0.4 to 0.59, p <0.0001). The κ value for the concordance among the 3 platelet function tests to identify patients with HPR was 0.42.
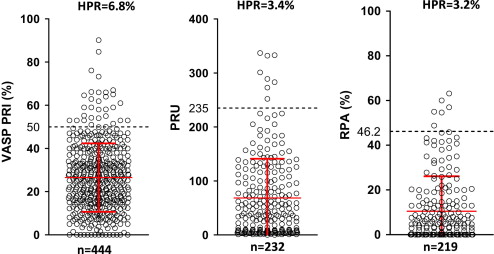
Obese patients (body mass index >30 kg/m 2 ) exhibited higher VASP PRI levels compared to nonobese patients (33 ± 16% [n = 91] vs 25 ± 15% [n = 353], p <0.0001). Elderly patients (aged ≥75 years) had lower VASP PRI compared to younger patients (21 ± 15% [n = 54] vs 27 ± 15% [n = 390], p <0.0001). Patients with diabetes (n = 128) had higher VASP PRI compared to those without diabetes (28 ± 16% [n = 128] vs 25 ± 16% [n = 316], p = 0.01).
After backward multivariate logistic regression analysis, body mass index >30 kg/m 2 and 3-vessel disease were the only 2 factors associated with HPR ( Table 2 ).
| Variable | Univariate OR (95% CI) | p Value | Multivariate OR (95% CI) | p Value |
|---|---|---|---|---|
| Age ≥ 75 yrs | 0.47 (0.1–2.0) | 0.31 | — | |
| Male gender | 1.68 (0.5–5.7) | 0.41 | — | |
| Low weight (<60 kg) | 2.43 (0.3–18.5) | 0.4 | — | |
| Body mass index >30 kg/m 2 | 2.71 (1.3–5.8) | 0.01 | 2.96 (1.4–6.4) | 0.006 |
| Hypertension (presence) | 1.1 (0.5–2.2) | 0.864 | — | |
| Diabetes (presence) | 1.39 (0.6–3.0) | 0.4 | — | |
| Dyslipidemia (presence) | 0.83 (0.4–1.7) | 0.62 | — | |
| ST-segment elevation myocardial infarction (presence) | 0.88 (0.4–1.8) | 0.72 | — | |
| 3-vessel disease vs 1- or 2-vessel disease | 2.3 (0.9–5.9) | 0.09 | 2.7 (1.02–7.15) | 0.045 |
| Creatinine clearance <60 ml/min | 0.64 (0.2–2.2) | 0.48 | — | |
| Proton pump inhibitor use | 1.2 (0.4–3.5) | 0.77 | — | |
| β-blocker use | 0.5 (0.2–1.1) | 0.09 | — |
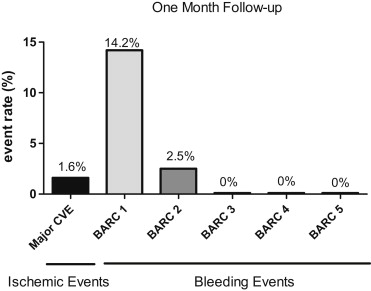
There were neither major bleeding complications (BARC grade 3) nor fatal bleeding (BARC grade 5) ( Figure 2 ). Because none of the patients underwent coronary bypass surgery, there was no BARC grade 4 bleeding (coronary artery bypass graft related). However, there was 14.2% (n = 63) BARC grade 1 bleeding (nuisance bleeding) and 2.5% (n = 11) BARC grade 2 bleeding (bleeding requiring a medical consultation; epistaxis n = 5, gastrointestinal bleeding n = 2, cutaneous bleeding n = 2). In the population of patients aged <75 years and/or weighing >60 kg, the rate of BARC grade 1 bleeding events did not differ significantly at 12.3% (n = 45) and 1.9% (n = 7) for BARC grade 2 bleeding.
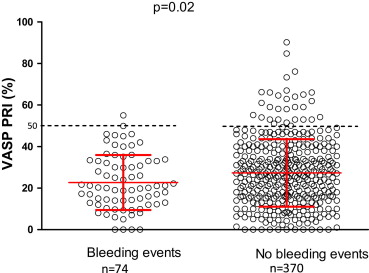
Platelet response is presented in Figure 3 . The area under the curve for the VASP test to predict bleeding events was low at 0.57 (95% CI 0.51 to 0.64, p = 0.03). The cutoff value of VASP 15.05% offered sensitivity of 35% and specificity of 77% to be associated with bleeding events.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


