Atrial septal defect (ASD) is one of the most common congenital cardiac defects, accounting for approximately 6% to 10% of all congenital cardiac defects. Transcatheter ASD closure has gradually matured as an alternative to surgical closure and has been employed increasingly in recent years, especially in cases of secundum ASD. The cardiac transcription factor NKX2.5 was identified as the first genetic cause of non-syndromic congenital heart disease and were shown to be associated with cases of familial ASD and atrioventricular (AV) conduction disturbances.
We report a patient who had plan of device closure of ASD but the device closure was not done because of second degree AV block in 24 hr ambulatory ECG monitorization.
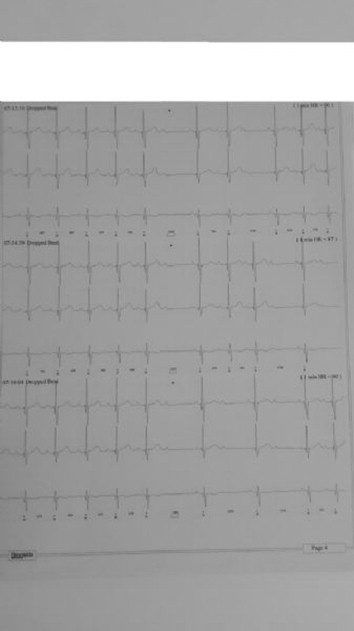
A 4-year-old girl presented with a history of fatigue and dizziness. In the family medical history, her aunt died suddenly due to unknown cardiac defect. Cardiovascular examination showed a regular pulse, fixed splitting of the second heart sound, and an ejection systolic murmur grade 2/6 at the upper left sternal border. In ECG she had normal sinus rhythm, mildly right-sided deviation of frontal axis, and evidence of incomplete right bundle branch block (RSR′ pattern in V1-V3).Transthoracic echocardiography showed a 10 mm ostium secundum ASD with adequate superior, posterior and inferior rims and dilated right atrium and right ventricle. Transcatheter device closure of ASD was planned for patient and 24 hr ambulatory ECG monitoring was made for further investigation of dizziness. She had Mobitz type I (Wenckebach) AV block in 24 hr Holter records (Figure 1). Therefore device closure of ASD was delayed and patient was directed to another center for genetic analysis of NKX2.5 mutations. Because specific types of these mutations result in progressive AV conduction disturbance, requiring pacemaker implantation.
The identification of NKX2.5 mutations in familial ASD patients is, therefore, of practical importance in that the existence of such mutations may predict the risk of progressive AV block and the requirement for pacemaker implantation during and after device closure.