Fig. 12.1
Roller pump
12.1.2.2 Centrifugal Pump
In plastic housing, a complex of plastic cones coupled magnetically to an electric motor. They rotate rapidly; by creating a pressure gradient between the inlet and outlet of the pump, kinetic energy is transferred to blood and resulting forward blood flow (Fig. 12.2). In spite of roller pump, centrifugal pump is non-occlusive; therefore flow rate is dependent to preload and afterload too.
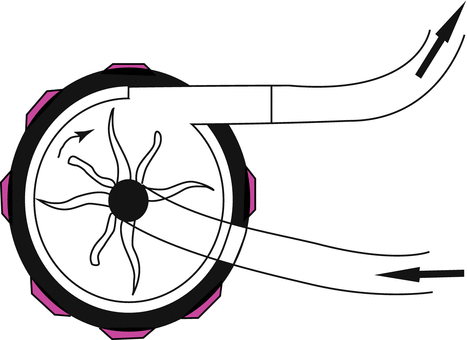

Fig. 12.2
Centrifugal pump
12.1.3 Cardiotomy
A filtered reservoir collects blood drained from the venous circulation. It is for storage, defoaming, and filtration before added directly to arterial circuit. Other fluid such as blood products and medication may also be added.
Reservoir designs include open or closed systems. The open system (hard shell) has graduated lines that shows amount of blood volume in the container. The design is open to atmosphere, allowing blood interface with atmospheric gases possible. In the closed system, the soft pliable bag eliminates the air–blood interface. Volume is measured by weight or by change in radius of the container.
12.1.4 Membrane Oxygenator
A flat sheet of hollow fibers imitates the pulmonary capillary function, by interposing a thin membrane between blood and gases, and creates gas–blood interface gas without blood passing. Gas flows through the hollow fiber and blood flow is around the fiber. In spite of carbon dioxide, oxygen is not diffusible in plasma well, so the blood is spread very thin to facilitate the transfer of oxygen by increased gradient pressure.
12.1.5 Anticoagulation
Unfractionated heparin is used to achieve adequate systemic anticoagulation, by measuring activated clotting time (ACT) ≥400 s. It remains the absolute choice of anticoagulant in cardiac surgery. Heparin is obtained from lung beef and or porcine intestinal mucosa. It isn’t an anticoagulant itself but could potentiate the antithrombin III (AT), a potent endogenous anticoagulant of body.
Heparin is given in the bolus dose of 2.5–4 mg/kg to aim an ACT of 400–480 s, before aortic and venous cannulation. Additional heparin is repeated to maintain ACT above 400 s during CPB, for adequate anticoagulation state.
An inadequate response to heparin (heparin resistance) may be seen in patients with long preoperative heparin use; this condition is corrected by FFP or recombinant AT administration.
At the end of operation, after CPB weaning it is reversed by protamine (isolated from fish sperm) to establish normal hemostasis condition after surgery. There are some ways to determine the protamine dose: protamine titration, fixed dose of protamine (in the ratio of 1.2–1.5 mg for 100 unit of heparin previously administered), ACT/heparin dose response curve, and heparin concentration. Protamine has mild anticoagulant effect too and inhibits platelet-induced aggregation. Although it has short half-life, but care should be taken not to infuse protamine too fast as it can induce systemic hypotension due to systemic vasodilation and pulmonary vasoconstriction.
Heparin rebound, a state of recurrent anticoagulant activity of heparin after adequate protamine administration, contributes in postoperative bleeding. Because not all of heparin is bounded to protamine, some of it binds to other plasma proteins and vascular cells, reappearance in circulation gradually.
12.1.6 Cannulation
Arterial and venous cannulation sites are influenced by the planned operation. In routine valve and coronary surgeries, the ascending aorta and right atrium are selected. Alternative sites of arterial cannulation are the femoral artery, axillary artery, and left ventricular apex. Cannulation site for venous access can be the inferior and superior vena cava, femoral vein, and internal jugular vein. Cannula is made from clear polyvinyl chloride, the oxygenator casing and connections are from polycarbonate.
12.1.7 Cardioplegia
A blood-free and motionless operative field is obtained by potassium-based cardioplegic solution that causes diastolic electromechanical arrest. In addition to stop electrical and consequently mechanical activation, preserving myocardial function and attenuating ischemic–reperfusion injury are other main goals of it. Cardioplegic solution can be categorized according to the type of solution (crystalloid vs. bloody), temperature (cold vs. warm), infusion type (antegrade into aortic root vs. retrograde through coronary sinus), and infusion interval (continuous vs. intermittent).
12.1.8 Heat Exchanger
The heat exchanger is used in combination with the oxygenator. This device is typically placed just before the oxygenator, because it helps to prevent bubble forming in the blood too. Heat exchanger controls the body temperature by heating or cooling blood passing through the circuit, at the beginning and end of CPB, respectively. In heat exchanger, the blood and water lines are separated by a metallic barrier. As the water temperature is changed, the blood temperature which enters the body circulation and the tissue temperature change. There is a consensus on protective effect of hypothermia for organ function during ischemic period. Hypothermia reduces oxygen consumption and metabolic rate. Even mild hypothermic condition can increase brain tolerance against ischemic injury. Although it is associated with side effects such as induced coagulopathy and leftward shift of oxygen–hemoglobin dissociation curve. Acid–base balance is affected by hypothermia, too. Depending on the type of operation, the patient’s temperature may be kept normothermic to less than 20 °C. Monitoring the blood temperature during the operation and the speed and temperature of rewarming is mandatory. Rewarming too great or too quickly may affect important problems. Hyperthermia during rewarming period is known to intensify ischemic damages especially in the brain and kidney.
12.1.9 Arterial Filter
Inclusion of arterial filter in CPB circuit is used to reduce microembolic events. It holds air bubble, particles of platelet aggregation, and thrombus during CPB.
12.1.10 Minimized Extracorporeal Circulation
Minimized extracorporeal circulation (MECC) is an alternative to conventional CPB. It consists of a membrane oxygenator, centrifugal pump, and arterial filter. It includes short heparin-coated circuit, less prime volume, and no cardiotomy suction and venous reservoir, thus the blood–air interface is limited, while conventional CPB circuit is an open circuit because of free contact of blood–air. The shed blood is washed through cell-saving device before return to arterial line. It seems that these differences lead to attenuate the adverse effect of conventional CPB: less inflammatory response, reduced hemodilution (and therefore less need to blood transfusion), and less changes in hemostasis system; some studies showed cardiac surgery with MECC is associated with less postoperative neurologic deficit, less need for blood transfusion, and less postoperative bleeding.
12.1.11 Ultrafiltration
Ultrafiltration removes water and low molecular weight materials from blood to a filtrate part under hydrostatic pressure through hollow-fiber semipermeable membrane. Ultrafiltration is used during cardiac surgery to reduce total body water, attenuate hemodilution during pump and need to transfusion, and remove inflammatory mediators. Hemodilution is inevitable at the beginning of CPB. There is a mixture of patients’ blood with prime content of CPB tube at the beginning of CPB, inducing acute hemodilution. Although hemodilution decreases blood viscosity and facilitates tissue perfusion in hypothermic setting, studies have shown that intraoperative hematocrit less than 23 % is associated with interstitial edema (decreased oncotic pressure) in vital organs and increased mortality; it decreases blood transfusion requirement. There are two main types of ultrafiltration. Conventional ultrafiltration is done only during CPB, and modified ultrafiltration is performed after CPB weaning to concentrate plasma volume.
12.2 Cardiopulmonary Bypass-Related Complications
12.2.1 Inflammation
Cardiopulmonary bypass and blood contact with synthetic surface of CPB circuit can promote whole-body systemic inflammatory response that plays a role in multiorgan failure. Continuous exposure of heparinized blood to foreign surfaces in the perfusion circuit and nonendothelial cells in the wound provoke complement anaphylatoxins, adhesion molecules, proinflammatory cytokines, vasoactive substances, coagulation, and fibrinolytic activation. In addition, ischemic–reperfusion injury (due to aortic clamp and declamping), endotoxemia (due to splanchnic hypoperfusion), hypothermia, surgical trauma, blood loss, and transfusion may contribute in activation of inflammatory cascade. Leukocyte activation, especially neutrophil, is responsible to initiate the release of inflammatory mediators that have negative impact in all organs. The severity of this exaggerated response can range from being barely clinically detectable to respiratory failure, coagulopathy, and multiorgan dysfunction. There are suggested strategies to attenuate inflammatory response, though there is not a consensus regarding their clinical outcome and benefits; these include heparin-coated circuit, corticosteroids, hemofiltration, leukocyte depletion, minimized extracorporeal circulation, aprotinin, complement inhibitors, free radical scavengers, and antioxidants (Miller and Levy 1997; Paparella et al. 2002; El Kebir et al. 2005; Raja and Dreyfus 2005; Patel and Ghatak 2008; Warren et al. 2009a, b; Rimmele et al. 2010; Augoustides 2012; Dieleman et al. 2012; Hausenloy et al. 2012).
12.2.2 CPB and Hematologic Effect
All blood elements get impression during CPB due to activation of haemostatic–inflammatory system. Pump time duration and the influence of biomaterial substances in surface area of CPB have the most effect on the severity of humoral and cellular activation. In coagulation system, reduction in platelet count and function, consumption of coagulation factors and increased activity in fibrinolytic field occur. Blood contact with artificial surfaces of CPB circuit makes intrinsic coagulation pathway activation. Extrinsic coagulation pathway is activated in pericardial blood, which is usually aspirated and returned to pump circulation. In addition to mechanical trauma to red blood cell, mixture of blood with priming solution brings to a dilutional anemia in the beginning of CPB. There are strategies to decrease this response: heparin coating of CPB circuit, corticosteroids, leukocyte filter, and ultrafiltration (Chung et al. 1996).
12.2.3 CPB and Kidney
Prevalence of acute kidney injury (AKI) after cardiac surgery has been reported between 1 and 30 %, according to definitions used for AKI, as there is no consensus in definition. It is associated with significant short- and long-term mortality. After cardiac surgery, only minimal changes of serum creatinine as small as 0.3 mg/dL predict prognosis in this setting, even it remains in normal range too; so attention to preventable causes, early detection, and treatment are among the valuable issues for prevention of AKI after cardiac surgery (Ronco et al. 2002; Boldt et al. 2003; Lassnigg et al. 2004; Karkouti et al. 2005; Mitter et al. 2010; Moriyama et al. 2010; Park et al. 2010b; Mariscalco et al. 2011; Weir et al. 2011; Ko et al. 2012; Kohagura and Ohya 2012; Sviglerova et al. 2012; Tolpin et al. 2012).
The most important causes of AKI during CPB (which overlap and interact at the cellular level):
1.
Hemodynamic insults (perioperative reduction of cardiac output and resultant decreased renal perfusion).
2.
Inflammatory factors (contact activation of blood components).
3.
Embolic events (both gaseous and particulate).
4.
Preexisting kidney dysfunction.
5.
Nephrotoxic agents (medications and intravenous contrast); in addition, type of operation has an effect on AKI. Among adult cardiac surgeries, coronary artery bypass graft (CABG) has the lowest incidence of AKI, while the combination of CABG and valve surgery is the highest risk factor.
CPB per se is responsible for renal injury duo to non-pulsatile flow. Other factors that are related to CPB include duration of CPB time and cross-clamp time, hemolysis, and hemodilution.
1.
Time duration of CPB and cross-clamp: The consequences of prolonged CPB are extension of hypoperfusion time and release of more inflammatory mediators, as it is directly correlated to CPB duration and disturb tubular renal function.
2.
Hemolysis: Hemolysis is the result of prolonged CBP time, cardiotomy suction, occlusive roller pump, blood exposure to artificial surface, and shear forces.
3.
Hemodilution: Because of need to priming in CBP tubes, hemodilution is inevitable; it is suggested that hemodilution improves regional blood flow (by reduction of blood viscosity) in the setting of hypothermia and hypoperfusion; although it was thought that improvement in regional blood flow compensates the risk of acute anemia (loss of O2-carrying capacity of blood), recent studies expressed that intraoperative hematocrit ≤21 % is an independent risk factor for postoperative AKI.
During operation modifiable factors that reduce the risk of AKI are prevention of excessive hemodilution, shorter duration of CPB, optimal glucose control, and use of NaHCO3. In practice kidney function is assessed by measuring serum creatinine, but it has limitations. Rise in serum creatinine indicates the reduction of glomerular filtration rate and occurs slowly in several days after proved injury. So it is an unreliable and insensitive indicator during early stages of kidney injury. Multiple studies have suggested that NGAL (neutrophil gelatinase-associated lipocalin) is a sensitive biomarker that shows tubular cell damage as early as 2 h after the event. It can be used as a reliable marker to diagnosis of acute kidney injury in post-cardiac surgery (Ronco et al. 2002; Boldt et al. 2003; Lassnigg et al. 2004; Karkouti et al. 2005; Mitter et al. 2010; Moriyama et al. 2010; Park et al. 2010a, b; Mariscalco et al. 2011; Weir et al. 2011; Bayrak et al. 2012; Ko et al. 2012; Kohagura and Ohya 2012; Levi et al. 2012; Sviglerova et al. 2012; Tolpin et al. 2012; Vellinga et al. 2012; Yap and Lee 2012).
12.2.4 CPB and Lung
Pulmonary dysfunction and prolonged ventilation after cardiac surgery are important causes of morbidity. It occurs due to combined effects of anesthesia, surgical trauma, and CPB. Causative factors of pulmonary impairment function during cardiac surgery are:
1.
Pulmonary ischemia
2.
Reperfusion injury
3.
Surgical trauma
4.
Hypothermia
5.
Blood loss and blood contact with artificial surfaces of CPB circuit
Lack or reduced blood flow through the lungs during surgery increases the damage of other factors. As the lung perfusion is limited to bronchial arterial system during aortic cross-clamp period. The clinical pattern of this injury has a range from subclinical functional changes to ARDS, due to lung edema, atelectasis, increased alveolar–arterial oxygen gradient, abnormal gas exchange, increased intrapulmonary shunting, and decreased lung compliance. Studies showed that neutrophil accumulation in lung during CPB, its activation, release of chemical mediators, and proteolytic enzymes are in charge of lung injury. In addition to inflammation response, volume overload during CPB plays an important role in accumulation of interstitial fluid in lung too. It was shown that prolonged CPB time, aortic valve surgery, and combined valve/CABG procedures are independent factors of postoperative respiratory failure. There are some therapeutic intervention to prevent or treat pulmonary dysfunction, such as corticosteroid administration, leukocyte depletion, heparin-coated circuit, hemofiltration, maintaining pulmonary ventilation during CPB, maintaining pulmonary perfusion during CPB, pump time reduction, and use of minimized CPB (Carvalho et al. 2008; Filsoufi et al. 2008; Goebel et al. 2008; Suzuki 2010).
12.2.5 CPB and CNS
Neurologic abnormalities such as stroke (prolonged or permanent deficit) and post- operative cognitive dysfunction are relatively common problems despite improvement in anesthetic and surgical techniques. They are important and disturbing complications after cardiac surgery. The independent predictors of postoperative stroke are:
1.
Peripheral vascular disease
2.
Diabetes mellitus
3.
History of cerebrovascular disease
4.
High transfusion requirement
5.
Urgent operation
6.
Left main coronary disease
7.
Carotid bruits
CPB-related mechanisms include:
1.
Macro- or microemboli (gas, atheromatous plaque, inorganic, and biologic debris from open cardiac procedures)
2.
Systemic inflammatory response
3.
Perioperative hypoperfusion
4.
Extensive aortic calcification
5.
Prolonged CPB time
6.
Hemorrhage
7.
Cerebral hyperthermia
The majority of strokes are due to embolic event, and watershed component is seen in few patients; the most atheromatous embolic event happens during manipulation of atherosclerotic aorta (cannulation, cross-clamp, and declamping), and its consequences are crack or rupture of atherosclerotic plaque leading to debris release, cerebral embolization, and ischemic brain injury. Aortic clamping in the presence of exophytic plaque is contraindicated, and elimination of CPB is recommended in this condition. It is suggested that epiaortic ultrasonography be used to identify atheromatous plaque and help to select the suitable site for aortic cannulation in the diseased ascending aorta. It is more sensitive than digital palpation and transesophageal echocardiography and gives information about the entire length of the ascending aortic wall; it can affect the rate of stroke whenever operative strategies are changed. Prevention of microemboli is accomplished by the use of a membrane oxygenator, the prevention of air entering the circuit, and the use of a microfilter that will effectively trap microemboli and prevent from reentering the blood stream and preventing the manipulation of diseased aorta. Prolonged period of hypoperfusion is a predisposing factor of ischemic events especially in old, diabetic, and hypertensive patients who have impaired autoregulatory mechanism in cerebral circulation; maintaining high perfusion pressure is recommended in these patients during CPB. Cerebral oximetry monitor can be used to assess cerebral perfusion during cardiac surgery. Decrease and increase in temperature of brain (during cooling and rewarming period) happens more quickly than other parts of the body because of the well- perfused state. Rapid rewarming at the end of CPB can lead to cerebral hyperthermia and exacerbated neurologic dysfunction. During complex aortic arch surgery, neurologic complication is associated less in antegrade cerebral perfusion (through right axillary artery cannulation) than retrograde cerebral perfusion and deep hypothermic circulatory arrest; in these patients femoral artery cannulation has the risk of retrograde cerebral embolization and worse neurologic outcome. Those who experience stroke during 24 h of CPB had adverse outcome in contrast to late presentation, although the majority of strokes happen after initial normal neurologic recovery of CPB, delayed stroke. Cognitive disorder such as a decrease in baseline performance is more frequent than neurologic deficit by the same mechanisms. Mild hypothermia is recommended for the reduction of postoperative cognitive disorder (Hogue et al. 1999, 2007, 2008a, b; Puskas et al. 2000; Salazar et al. 2001; Ascione et al. 2002; Bucerius et al. 2003; Bergman et al. 2004; Carrascal et al. 2005; Zingone et al. 2006; Biancari et al. 2007; Nathan et al. 2007; Campos and Paniagua 2008; Gottesman et al. 2008; Grogan et al. 2008; Lisle et al. 2008; Tully et al. 2008; Grigore et al. 2009; Slater et al. 2009; Coburn et al. 2010; Lombard and Mathew 2010; Rudolph et al. 2010; Hedberg et al. 2011; El Zayat et al. 2012; Messerotti Benvenuti et al. 2012a, b; Misfeld et al. 2012; Sanders and Grocott 2012; Sun et al. 2012; Bartels et al. 2013; Bruggemans 2013; Reinsfelt et al. 2013).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


