Anticoagulant
Half-life (t1/2)/dose
Monitoring
Discontinue prior to surgery (days)/reversal agent
Dabigatran (Pradaxa®). https://www.pradaxa.com/. Accessed 17 July 2012
12–17 h in healthy subjects
No readily available method
CrCl ≥50 mL/min: 1–2 days
CrCl >30 mL/min: 150 mg BID
Activated partial thromboplastin time (aPTT) demonstrates presence but not degree of anticoagulation
CrCl 30–50 mL/min: 2–4 days
CrCl 30–50 mL/min + dronedarone or ketoconazole: 75 mg BID
Prothrombin time (PT) insensitive
CrCl <30 mL/min: ≥5 days
CrCl 15–30 mL/min: 75 mg BID
Thrombin time (TT)—normal value rules out presence of dabigatran
Ecarin clotting time (ECT)—linear dose relationship; not routinely available
Rivaroxaban (Xarelto®). http://www.xareltohcp.com/. Accessed 17 July 2012
5–9 h in healthy subjects
No readily available method
At least 1 day (24 h)
Atrial fibrillation
Prolongs aPTT, PT/INR
No reversal agent available and unlikely to be dialyzable due to high protein binding (Ageno et al. 2012)
CrCl >50 mL/min: 20 mg daily with evening meal
No direct effect on platelet aggregation
CrCl 15–50 mL/min: 15 mg daily with evening meal
VTE prophylaxis: 10 mg daily ± food
VTE treatment: 15 mg PO twice daily × 21 days, then 20 mg daily
Apixaban (Eliquis®). http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed 27 February 2013
~12 h following repeated dosing
No readily available method
24–48 h prior to surgery depending on risk, location, and ability to control bleeding
Atrial fibrillation
Prolongs aPTT, PT/INR
No reversal agent and unlikely to be dialyzable due to high protein binding
5 mg twice daily
No direct effect on platelet aggregation
Activated charcoal may be useful in overdose situations
2.5 mg twice daily in patients with any two of the following characteristics: ≥80 years of age, weight ≤60 kg, serum Cr ≥1.5 mg/dL
2.5 mg twice daily in patients taking strong dual inhibitors of CYP3A4 and P-gp (ketoconazole, itraconazole, ritonavir and clarithromycin)
No data in patient with CrCl <15 mL/min
Warfarin (Coumadin®). http://packageinserts.bms.com/pi/pi_coumadin.pdf. Accessed 17 July 2012
20–60 h
PT/INR
Minimum of 5 days without reversal agents
Individualized dosing
Reversal agents
Vitamin K
10 mg PO/IVPB for emergent normalization of PT/INR; IVPB initial effect at 2 h and full correction within 24 h
5 mg PO and 1 mg IVPB produce similar effects on INR at 24 h
0.5–1 mg orally for reducing PT/INR into therapeutic range (for <2.5 mg use IV form administered orally)
Ineffective in hepatic disease due to inability to produce factors
Oral route not effective in biliary disease
SQ not recommended due to unpredictable absorption and reversal characteristics
Prothrombin complex concentrate (PCC, Factor IX complex, Profilnine®)
Recombinant activated factor VII
For intracranial hemorrhage—doses vary; 20–40 μg/kg have been used; available as 1, 2, 5, and 8 mg vial sizes; use lowest dose rounded to nearest vial size and repeat if needed due to risk of arterial and venous thrombotic and thromboembolic events
6.2.2 Parenteral Anticoagulants
Unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), direct thrombin inhibitors, and indirect factor Xa inhibitors are parenteral agents with varying dosing schedules and clinical uses. Heparin and LMWH are used for the treatment of acute coronary syndromes (ACS), VTE, and for prophylaxis of thrombotic events. Both UFH and LMWH indirectly inhibit factor IIa and Xa, with LMWH having a higher affinity for factor Xa. Fondaparinux is an indirect factor Xa inhibitor used for treatment and prophylaxis of VTE. Direct thrombin inhibitors (DTIs) are used in the treatment of heparin-induced thrombocytopenia (HIT) and include argatroban and bivalirudin (Angiomax®). Of the available DTIs, bivalirudin has been used in patients with a history of HIT requiring cardiac surgery. Of the agents listed in Table 6.2, heparin and the DTIs are the primary parenteral anticoagulants in the cardiac surgery setting and will therefore be the focus in this section.
Anticoagulant | Half-life (t1/2)/dose | Monitoring | Discontinue prior to surgery (hours)/reversal agent |
|---|---|---|---|
Unfractionated heparin (UFH) | 60–90 min | aPTT | 4–6 h |
VTE: 80 unit/kg bolus, then 18 units/kg/h | Anti-factor Xa activity level (UFH levels) | Protamine 1 mg/100 units of heparin (max 50 mg at a rate not to exceed 5 min) | |
ACS: 60 unit/kg bolus, then 12 units/kg/h | Activated clotting time (ACT; intraoperatively) | Dose adjust based on time since heparin held: >60 min 0.5 mg/100 units; >2 h 0.25 mg/100 units | |
Prophylaxis: 5,000 units SQ BID or TID | |||
Low-molecular-weight heparin | 4.5–7 h | Anti-factor Xa activity level (LMWH level) | 24 h |
Dalteparin (Fragmin®). www.pfizer.com/files/products/uspi_fragmin.pdf. Accessed 18 July | VTE treatment | Dalteparin treatment doses should not be used in patients with CrCl ≤30 mL/min | Protamine |
Dalteparin: 200 units/kg SQ daily | Enoxaparin 1 mg/kg SQ daily may be considered in patients with chronic stable kidney disease and CrCl ≤30 mL/min who are not dialysis dependent; anti-factor Xa and serum creatinine monitoring is highly recommended | <8 h after last dose: 1 mg/1 mg enoxaparin or per 100 units of dalteparin | |
Enoxaparin: 1 mg/kg SQ BID or 1.5 mg/kg SQ daily | 8–12 h after last dose or if repeat is necessary: 0.5 mg/1 mg enoxaparin or per 100 units of dalteparin | ||
Enoxaparin (Lovenox®). http://products.sanofi.us/lovenox/lovenox.html#section-14.1. Accessed 18 July | VTE prophylaxis | >12 h after last dose: administration of protamine may not be necessary | |
Dalteparin 5,000 units SQ daily | The anti-factor Xa activity is never completely reversed (typically 60 % is reversed) | ||
Enoxaparin 30 mg SQ BID or 40 mg SQ daily | |||
ACS | |||
Dalteparin 120 units/kg SQ every 12 h | |||
Enoxaparin 1 mg/kg SQ every 12 h | |||
Direct thrombin inhibitors | Argatroban 50 mins | aPTT | 2 h |
Argatroban®. http://us.gsk.com/products/assets/us_argatroban.pdf. Accessed 31 July 2012 | Treatment of HIT: 2 mcg/kg/min initial dose; adjust for hepatic insufficiency and critically ill patients with multisystem organ failure | ||
Bivalirudin (Angiomax®). www.angiomax.com/Downloads/Angiomax_PI_2010_PN1601-12.pdf. Accessed 18 July 2012 | Bivalirudin 25 mins | No reversal agent | |
CPB dosing in setting of HIT | |||
On pump:1 mg/kg bolus, 50 mg for pump then 2.5 mg/kg/h; goal ACT >2.5x baseline | Case reports suggest that recombinant factor VIIa 90 μg/kg × 1 may reverse the anticoagulant effect (Schulman and Bijsterveld) | ||
Off pump: 0.75 mg/kg bolus, 1.75 mg/kg/h: goal ACT >300 s | |||
Factor Xa inhibitor Fondaparinux (Arixtra®). http://us.gsk.com/products/assets/us_arixtra.pdf. Accessed 31 July 2012 | 17–21 h | Not routinely available. International standards for anti-factor Xa activity for UFH/LMWH do not apply | 48 h |
VTE | No reversal agent | ||
Prophylaxis: 2.5 mg SQ daily | Case reports suggest that recombinant factor VIIa 90 μg/kg × 1 reverse the anticoagulant effect (Schulman and Bijsterveld) | ||
Treatment: | |||
<50 kg: 5 mg SQ daily | |||
50–100 kg: 7.5 mg SQ daily | |||
>100 kg: 10 mg SQ daily |
Heparin is the anticoagulant of choice in the cardiac surgery setting due to its fast onset/offset, reversibility with protamine, and point of care (POC) monitoring option. Benefits during CPB include inactivation of thrombin and factor Xa thereby reducing the risk of clot formation in the circuit. Typical doses include 20,000–30,000 units to achieve a target ACT of 300–350 s, although this varies widely among cardiac surgery centers. Rarely, patients may require doses of heparin exceeding 500 units/kg without achieving the target ACT. These patients may have a reduced response to heparin due to low levels of antithrombin (AT), excessive factor VIII, or excessive fibrinogen (Dager 2011). Antithrombin is a required cofactor for the anticoagulant effect of heparin. Risk factors for antithrombin deficiency include preoperative heparin, placement of an intra-aortic balloon pump (IAPB), elevated platelet counts, and infective endocarditis (Ranucci et al. 1999). Repletion of AT and subsequent goal ACT can be achieved with administration of fresh frozen plasma (FFP) or commercially available concentrates (i.e., Thrombate®) (Lemmer and Despotis 2002).
Argatroban and bivalirudin are DTIs and are the primary agents used in the treatment of HIT. Bivalirudin has been used as the intraoperative anticoagulant in patients with a history of HIT or those who require surgical intervention in the presence of active HIT.
6.2.3 Antiplatelet Agents
Antiplatelet therapy is a cornerstone of primary and secondary prevention of cardiovascular disease in patients with a history of myocardial infarction, ACS, and some valvulopathies. Thienopyridines (clopidogrel and prasugrel) are hepatically or enzymatically metabolized to active metabolites which bind to the platelet P2Y12 receptor causing irreversible platelet inhibition. Antiplatelet therapy should be evaluated perioperatively with respect to dose, duration, last dose administered, and hemorrhagic side effects.
Aspirin irreversibly inhibits platelet cyclooxygenase-1 (COX-1) which prevents production of thromboxane A2. This results in inhibition of platelet activation and aggregation. At any given point, the age of circulating platelets ranges from 1 to 10 days. New platelets with functioning COX-1 are continually being produced and replace 10–15 % of inhibited platelets daily allowing overall platelet function to return gradually over a period of 7 days (Gibbs et al. 2001).
Clopidogrel is a prodrug hepatically metabolized in two-step process to an active form which inhibits ADP binding to the platelet P2Y12 receptor. This results in inhibition of ADP-mediated activation of the glycoprotein IIb/IIIa (GP IIb/IIIa) complex and resultant platelet aggregation. The amount of the active form in circulation varies due to genetic variation in hepatic metabolism (CYP2C19) and drug interactions affecting CYP2C19 (i.e., proton pump inhibitors). These factors may contribute to the variable response associated with this agent. Data evaluating the concurrent administration of omeprazole demonstrated no clinically relevant interaction; however, the manufacturer recommends the use of pantoprazole due to less inhibitory effect on CYP2C19 (Wallentin 2009; Wallentin et al. 2009; Patrono et al. 2011).
Prasugrel is a prodrug similar to clopidogrel and exhibits antiplatelet effects by the same mechanism. However, it is unaffected by polymorphisms in CYP2C19 or interactions with proton pump inhibitors. Additionally, the active component is produced following a one-step hepatic conversion resulting in a greater amount of metabolite formed following a loading dose. Prasugrel is 10x more potent than clopidogrel with a more rapid onset (30 min vs 1 h with 600 mg loading dose of clopidogrel) and less variation in response (Eikelboom et al. 2012; Patrono et al. 2011).
Ticagrelor undergoes enzymatic degradation to an active metabolite. Onset of action is approximately 30 min post loading dose of 180 mg (Weitz et al. 2012).
Intravenous antiplatelet agents administered primarily during percutaneous coronary intervention (PCI) include abciximab, eptifibatide, and tirofiban. These agents block the binding of fibrinogen to the GP IIb/IIIa receptor and subsequent platelet aggregation. Abciximab is a monoclonal antibody which acts as a noncompetitive inhibitor while eptifibatide and tirofiban are competitive inhibitors and therefore require high plasma levels for adequate antiplatelet effect (Patrono et al. 2011; Bojar 2011) (Table 6.3).
Table 6.3
Antiplatelet agent properties
Antiplatelet agent
Mechanism of action/dose
Duration of effect
Discontinue prior to surgery (days)
Oral agents
Aspirin
Cyclooxygenase inhibitor
7 days—affected platelets must be replaced
3–5 days depending on residual aspirin effect desired
81–325 mg daily
Clopidogrel (Plavix®). http://www.plavix.com/Index.aspx. Accessed 8 August 2012
Irreversible ADP-P2Y12 receptor inhibitor
7 days—affected platelets must be replaced
5–7 days
Loading dose (LD): 300–600 mg PO × 1; omit in patients ≥75 years of age
Maintenance dose (MD): 75 mg PO daily
Prasugrel (Effient®). http://www.effient.com/Pages/index.aspx. Accessed 8 August 2012
Irreversible ADP-P2Y12 receptor inhibitor
7 days—affected platelets must be replaced
7 days
LD: 60 mg PO × 1
MD: 10 mg PO daily; reduce to 5 mg PO daily in patients < 60 kg
Ticagrelor (Brilinta®). http://www.brilinta.com/. Accessed 8 August 2012
Reversible ADP-P2Y12 receptor inhibitor
48 h (t1/2 6–13 h including active metabolite) (Wallentin 2009)
3–5 days
LD: 180 mg × 1
MD: 90 mg PO BID
Intravenous agents
Abciximab (Reopro®). http://www.reopro.com/Pages/index.aspx. Accessed 8 August 2012
Irreversible glycoprotein IIb/IIIa inhibitor
24 h
24 h
PCI: 0.25 mg/kg bolus followed by infusion of 0.125 μg/kg/min × 12 h
PCI within 24 h: 0.25 mg/kg bolus followed by infusion of 10 μg/kg/min concluding 1 h post PCI
Eptifibatide (Integrilin®). http://www.integrilin.com/integrilin/index.html. Accessed 8 August 2012
Reversible glycoprotein IIb/IIIa inhibitor
4 h
4 h
180 μg/kg bolus followed by infusion of 2 mcg/kg/min
CrCl <50 mL/min:180 μg/kg bolus followed by infusion of 1 mcg/kg/min
Tirofiban (Aggrastat®). http://www.aggrastat.com/. Accessed 8 August 2012
Reversible glycoprotein IIb/IIIa inhibitor
4 h
4 h
0.4 μg/kg/min × 30 mins, then 0.1 μg/kg/min
CrCl <30 mL/min: reduce dose by 50 %
6.3 Other Agents
Supplements such as fish oil, vitamin E (>400 units/day) and herbal supplements can significantly impact coagulation and platelet function and should be discontinued at least 5 days prior to surgery. Examples of herbal supplements with anticoagulant or antiplatelet activity include chamomile, garlic, chondroitin, evening primrose oil, ginger, feverfew, ginseng, kava, mate, and goldenseal (Halasynski et al. 2004).
6.4 Monitoring Coagulation Status
The final product of a functioning coagulation cascade is the dynamic harmony between clot formation and dissolution known as hemostasis. Achieving hemostasis involves not only blood factors and platelets but also proteins (i.e., cytokines) and non-platelet cells (i.e., endothelial cells). Instability in the coagulation system due to interaction between these factors, often released in situations of vascular injury (i.e., surgery) or various illnesses (i.e., malignancy), increases the risk of hemorrhage or thrombosis. Within the coagulation system are two pathways known as the extrinsic pathway and the intrinsic pathway. Pathway function can be assessed by common laboratory tests (Table 6.4).
Assay/test | Normal range/values | Pathway/factors assessed | Disease states affecting results |
|---|---|---|---|
Plasma assays | |||
Prothrombin time/international normalized ratio (PT/INR) | INR 2–3.5 (Higher goals may be indicated in patients with hypercoagulability disorders) | PT/INR: Factors II, V, VII, and X (extrinsic and common pathways) | Coagulation disorder (hereditary or acquired factor VII, X, V, and II deficiencies ↑ INR) |
Medications | |||
Warfarin, rivaroxaban, dabigatran (↑ INR) | |||
Argatroban and daptomycin associated with falsely ↑ INR | |||
Factor deficiency due to hepatic disease, malabsorption, malnutrition, and chronic antibiotic therapy (PT/INR in patients with cirrhosis may not reflect bleeding risk due to overall deficiency in both pro– and anticoagulant factors) (O’Conner 2009) | |||
Anti-phospholipid syndrome (APLS) ↑ INR | |||
Hemodilution ↓ factor concentration | |||
Activated partial thromboplastin time (PTT) | 1.5–2.5 upper limit of normal reference range (therapeutic aPTT corresponds to the above but varies by institution due to reagents and instrumentation) | aPTT: II, V, VIII, IX, X, XI (intrinsic and common pathways) | Coagulation disorder |
Hereditary or acquired factor XII, XI, IX, VIII, X, V, and II deficiencies, APLS ↑ aPTT | |||
Medications which ↑ aPTT | |||
Rivaroxaban, DTIs, hydroxyethyl starch (HES) | |||
Anti-factor Xa activity assay | Range varies based on indication | Amount of heparin or LMWH in specimen via factor Xa inhibition | Substantially fewer uncontrolled variables versus PTT monitoring |
Treatment of VTE with UFH: 0.3–0.7 units/mL | Independent of other coagulation factors in the intrinsic pathway (Winkler and Zimring 2009) | ||
Treatment of VTE with LMWH twice daily dosing: 0.5–1 units/mL | |||
LMWH daily dosing: 1–2 units/mL | |||
Whole blood assays | |||
Activated clotting time (ACT) | Goal ranges vary for CPB (i.e., 300–450 s) | Measures intrinsic pathway factors | Used with high doses of heparin (i.e., during PCI or CPB) when aPTT is insensitive |
Prolonged by | |||
Hemodilution | |||
Heparin | |||
Hypothermia | |||
Hyperfibrinogenemia | |||
Thrombocytopenia | |||
Thromboelastography (TEG®/Rotem®) | Evaluates viscoelastic properties of whole blood and is unaffected by hypothermia (normal values vary by institution) | Intrinsic, extrinsic, common pathways, and platelet function | Most valuable in the bleeding patient vs preoperative prediction of bleeding |
Reaction time (R)—time to first fibrin strand formation (6–8 min) | May aid in distinguishing medical from surgical bleeding | ||
Clot formation time (K)—time for fibrin/platelets to initiate cross-linking (3–6 min) | TEG results must always be considered in conjunction with the clinical picture | ||
Alpha angle (α angle)—speed at which solid clot forms (50°–60°) | ↑ R/K—hypocoagulability related to factor deficiency or heparin/warfarin therapy | ||
Maximum amplitude (MA)—absolute strength of fibrin clot which is dependent on fibrin and platelets (50–70 mm) | ↓ R/K plus ↑ MA/α —hypercoagulability | ||
Clot lysis index (CLI)—loss of clot due to lysis 60 min after MA (85–100 %) | ↓ CLI—hyperfibrinolysis | ||
(Sie and Steib 2006) | |||
Thromboelastography, via either inpatient laboratory or point-of-care reporting, is becoming more widely available. This type of monitoring provides a graphic representation of a patient’s complete coagulation status. Tracings demonstrating hemorrhagic and thrombotic states are shown in Figs. 6.1 and 6.2
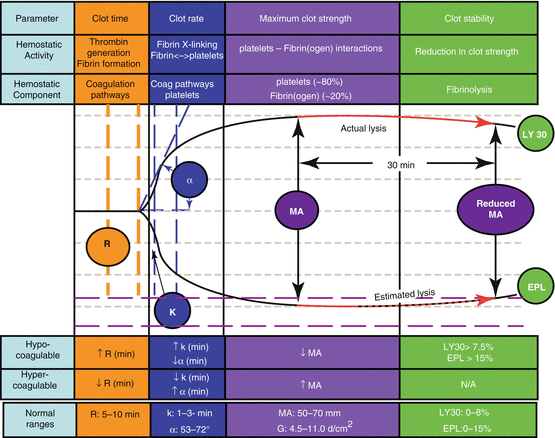
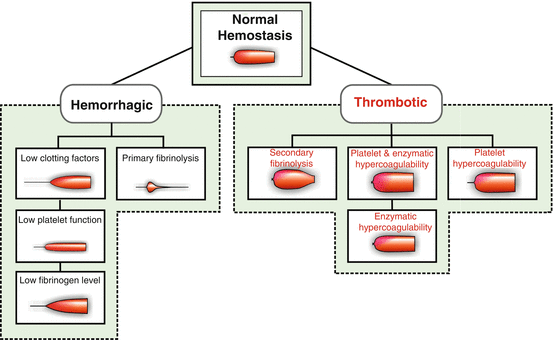

Fig. 6.1
Thromboelastograph parameters (Reproduced with permission from Haemonetics Corporation, Braintree, MA)

Fig. 6.2
Thromboelastograph tracing patterns for hemorrhagic and thrombotic conditions (Reproduced with permission from Haemonetics Corporation, Braintree, MA)
6.5 Postoperative Considerations
6.5.1 Medications
Heparin is the primary anticoagulant of concern in the immediate postoperative period. Initial reversal with protamine is performed in the operating room with a variety of dosing regimens (i.e., 1:1 or 0.5:1 ratio to the dose of heparin, dose based on ACT). Residual heparin can redistribute following reversal in the operating room (“heparin rebound”) requiring additional doses of protamine in the postoperative care area (i.e., 25 mg × 2 doses). Given that the elimination of protamine from the circulation occurs within 30 min (t1/2 = 5 min) from a bolus dose, a low-dose postoperative infusion of 25 mg/h for 6 h is an alternative dosing regimen that has demonstrated a reduction in postoperative bleeding, but not transfusion rate (Teoh et al. 2004). Conservative dosing will avoid the anticoagulant effect associated with high-dose protamine (Bojar 2011).
Following hemostasis postoperatively and depending on the procedure performed (coronary artery bypass, valve replacement, etc.), a variety of anticoagulant agents may be recommended to decrease the risk of postoperative VTE. Timing of initiation is patient-specific and dependent on a variety of factors including risk of bleeding/thrombosis, efficacy/tolerance of therapy preoperatively, and preoperative/preexisting thrombotic risk factors. Options include UFH 5,000 units subcutaneously (SQ) twice or three times daily, enoxaparin SQ 40 mg daily or 30 mg twice daily, or dalteparin 5,000 units SQ daily. In patients in whom pharmacologic VTE prophylaxis is deemed too risky, sequential compression devices should be initiated preoperatively.
6.5.2 Coagulopathies
Various coagulopathies (either transient or chronic) can complicate postoperative management. Prompt initiation/resumption of anticoagulant/antiplatelet therapy postoperatively may decrease risk of thrombosis (i.e., stent thrombosis). Table 6.5 describes the most common hypercoagulopathic states (Dager 2011; Bojar 2011).
Table 6.5
Prothrombotic coagulopathies
Syndrome | Pathology | Considerations |
|---|---|---|
Antiphospholipid syndrome (APLS) | Autoantibodies bind phospholipids | Paradoxically elevated aPTT |
Anticardiolipin antibody | Intraop: ACT may not be reliable; consider heparin-protamine titration with goal heparin of 3.4 units/mL; consider bivalirudin (Weiss 2008) | |
Anti-β2-glycoprotein I antibody | ||
Lupus anticoagulant | ||
Arterial and venous thrombosis | ||
Increased function/level of native procoagulants | Activated protein C resistance resulting in ↑ concentrations of activated factor V (factor V Leiden mutation) | Provide postoperative anticoagulation as soon as possible |
Prothrombin G20210A mutation resulting in ↑ concentrations of prothrombin | ||
↑ levels of factors VIII, IX, and XI | ||
Deficiencies of native anticoagulants | Protein C and S and antithrombin (AT) | AT deficiency: administer fresh frozen plasma or AT concentrate (Thrombate®) intraoperatively if using heparin |
Dosing based on AT level, clinical status, and setting | ||
Consider argatroban (AT independent) |
6.5.2.1 Disseminated Intravascular Coagulopathy
Disseminated intravascular coagulopathy (DIC) is an acquired hypercoagulable hematological complication resulting in activation of the coagulation system. Microthrombi deposit in various organs ultimately resulting in reduced blood supply and organ failure. Once circulating blood factors and platelets have been consumed, a hypocoagulable status prevails resulting in bleeding. Several disease states are associated with a risk of DIC and include sepsis, transplant rejection, severe hepatic failure, trauma, and hemolytic transfusion reactions (Franchini et al. 2006). Laboratory parameters associated with DIC are listed in Table 6.6.
Table 6.6
Laboratory parameters in DIC
Parameter
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|