Fig. 13.1
Graphical representation of the distinct posttranscriptional regulatory mechanisms operating during the transcription, splicing, editing, quality control checking, and maturation of mRNA transcripts
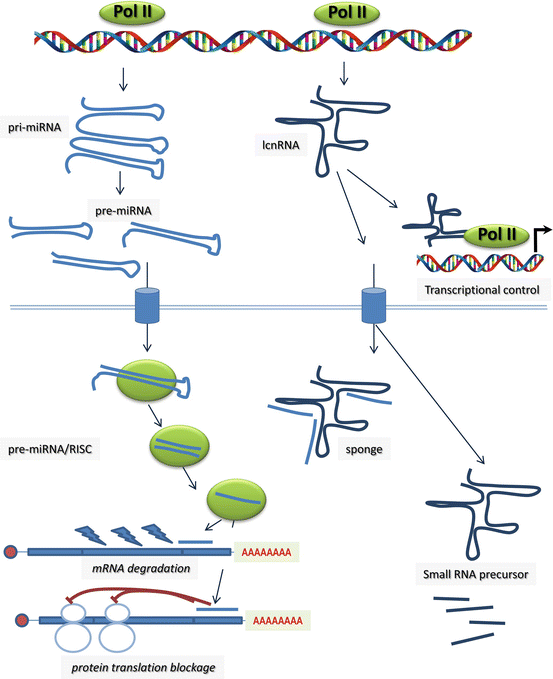
Fig. 13.2
Graphical representation of the microRNAs and long non-coding RNAs (lcnRNA) biosynthetic pathway and their functional roles during transcriptional and posttranscriptional regulation. microRNAs can elicit mRNA degradation and/or protein translation blockage. lncRNAs have been reported to actively contribute to transcriptional regulation and serve as sequestering small RNA system (sponge) or as template to generate smaller RNA molecules with, to date, poorly characterized functions
13.2.1 5′ End Capping
Eukaryotic mRNAs are modified by the addition of a 7-methylguanosine “cap” to the first transcribed nucleotide in the nucleus (Fig. 13.1). This modification is necessary for efficient gene expression and cell viability from yeast to humans. The 7-methylguanosine cap is required for transcription elongation, splicing, translation, and general mRNA stability. On the other hand, the 5′ cap seems to be required for polyadenylation and nuclear export of mRNA in S. cerevisiae [4], but not in metazoan cells [5, 6]. Several factors have been reported to regulate mRNA cap methylation in yeast [7]. Triphosphatases such as Cet1p and Pct1 direct the hydrolyzation of RNA 5′ triphosphate to a diphosphate-RNA. Guanylyltransferases, such as Ceg1p and Pch1, catalyze the addition of CMP to the diphosphate-RNA to produce the guanosine cap [8], while the methylation of the guanosine cap is mediated by Abd1 and Pcm1. In mammals, the triphosphate and guanylyltransferase activities are found within the same peptide [9, 10] the capping enzyme of RNA guanylyltransferase and 5′ triphosphatase (RNGTT), while the RNA methyltransferase (RNMT) is encoded by a distinct protein [9–13]. Interestingly, guanylyltransferase and methyltransferase are highly conserved in structure and function from yeast to humans, yet triphosphatases are widely divergent.
mRNA capping and cap methylation occur “co-transcriptionally,” that is to say, the cap methyltransferase is recruited to RNA polymerase II as the RNA is being transcribed, providing thus the means to promote transcription elongation [7]. Pre-RNA splicing is dependent on the 5′ cap since the splicing reaction has been demonstrated to be inhibited by the presence of free 7-methylguanosine [14]. The dependency of splicing on the 5′ cap is mediated by the cap-binding complex, which is a heteromeric complex formed by cap-binding protein (CBP) 80 and CBP20. From yeast to humans, the 5′ cap is necessary for the translation of almost all mRNAs, with the exception of mRNAs translated by an internal ribosome entry site [15]. The presence of a 5′ cap can also protect mRNA from degradation in X. laevis [16–18], while in S. cerevisiae inhibition of guanosine capping in vivo provoked rapid degradation in some but not all mRNAs, demonstrating the necessity for a guanosine cap to stabilize at a least a subset of mRNAs [4, 7, 19, 20]. Similarly, mRNA polyadenylation and nuclear export appear to largely be independent of the 5′ capping in S. cerevisiae [3] but dependent in other species such as X. laevis and humans [6, 21]. Thus, while the influence of 5′ capping is pivotal for subsequent mRNA biogenic steps such as transcriptional elongation, pre-mRNA splicing, and translation, species-specific differences seem to occur for degradation protection and mRNA polyadenylation. Given the essential role of 5′ capping in basal mRNA biogenesis, to date no specific defects affecting heart morphogenesis and/or muscle development have been reported.
13.2.2 3′ End Polyadenylation
Polyadenylation is a two-step nuclear process that involves an endonucleolytic cleavage of the pre-mRNA at the 3′-end and the polymerization of a poly-adenosine (polyA) tail (Fig. 13.1), which is fundamental for mRNA stability, nuclear export, and efficient translation during development [22]. The core molecular machinery responsible for the definition of a poly-A site includes several recognition, cleavage, and polyadenylation factors that identify and act on a given poly-A signal present in a pre-mRNA, usually an AAUAAA hexamer [22]. This mechanism is tightly regulated by both cis- and trans-acting factors, and its impairment can cause inefficient gene expression and thus disease. Previous studies have indicated that more than half of the human genes possess multiple polyadenylation sites [23], dubbed APA, which may produce mRNA isoforms with different protein-coding regions or 3′ UTRs of variable length. Interestingly, such a property is also documented in yeast [24]. The differential recognition of polyadenylation signals leads to long or short 3′ UTR of the transcripts. Usage of alternative poly(A) sites influences the fate of mRNAs by altering the availability of RNA-binding protein sites and miRNA binding sites. Abnormalities in the 3′-end processing mechanisms thus represent a common feature among many oncological, immunological, neurological, and hematological disorders [23, 25, 26], and the usage of APA and alterations in polyadenylation are beginning to be discovered and studied in human diseases [27, 28], yet to date no direct involvement in cardiovascular diseases has been reported.
13.2.3 Nonsense-Mediated Decay
Nonsense-mediated decay (NMD) is an evolutionary conserved surveillance pathway present in all eukaryotes studied to date. NMD plays an important role in the posttranscriptional control of gene expression. Approximately one-third of human genes generate pre-mRNAs that undergo alternative splicing, and similarly one-third of alternatively spliced transcripts are targeted for elimination by the NMD pathway [29]. Most alternatively spliced NMD targets appear to be generated in error [30], yet NMD also downregulates the level of other apparently normal transcripts [31–33]. NMD targets premature translation termination codons (PTC)-containing transcripts for rapid degradation (Fig. 13.1), thus protecting the organism from deleterious gain- or loss-of-function (dominant-negative effects) effects of the resulting truncated proteins [34–36]. As a rule, NMD degrades newly synthesized mRNAs during a pioneer round of translation [37–40] and occurs when a PTC is located more than 50–55 nucleotides upstream of the last exon-exon junction within the mRNA, and at least one intron and components of translation are present [41]. Importantly, there is a growing body of evidence supporting that mRNA decay in eukaryotes requires an exit from translation so that the mRNA is accessible to degradative activities [42–45].
The role of NMD in genetic diseases is emerging progressively. A pivotal role for NMD in cystic fibrosis as well as in Duchenne muscular dystrophy (DMD) has been documented (see for a review [46]), yet has only begun to be recognized in cardiac genetic diseases. Geiger et al. [47] recently reported that insufficient clearance of lamin A/C truncated mutations by NMD underlies the development of dilated cardiomyopathy in a human kindred. Similar findings have also been reported for nonsense mutations in hERG in the context of human long QT syndrome [48, 49]. Importantly an intricate relationship between NMD and the ubiquitin-proteasome system has been recently demonstrated in the context of hypertrophic cardiomyopathy [50], opening new ways to understand the complex RNA-protein interphase. In the context of congenital heart diseases, involvement of NMD has been proven for GATA binding protein 6 (GATA6) regulation in the setting of ventricular septal defect, patent ductus arteriosus, and congenital diaphragmatic hernia [51] and suspected in a kindred of syndromic patent ductus arteriosus as consequence the generation of aberrant transcription factor AP-2 beta (TFAP2B) splice variants [52].
13.3 mRNA Maturation: Generating Diversity (RNA Editing and Pre-mRNA Splicing)
13.3.1 RNA Editing
RNA editing relates to those molecular processes by which the RNA nucleotide sequence is conspicuously modified (Fig. 13.1). To date such changes have been observed in tRNA, rRNA, and mRNA molecules of eukaryotes, but not prokaryotes. RNA editing can modify an A-to-I (inosine) by the action of adenosine deaminase that acts on RNA (ADAR), and similarly a C-to-U modification can be elicited by a protein complex composed by APOBEC-1 (apolipoprotein B mRNA editing enzyme, catalytic polypeptide 1), an RNA cytidine deaminase, and APOBEC-1 complementation factor (ACF). This C to U editing holoenzyme (APOBEC-1/ACF) is also in part regulated by CELF2 [53, 54].
Inosine is an essential modification introduced by specialized enzymes in a highly regulated manner generating thereafter transcriptome diversity. Adenosine to inosine (A-to-I) modification by the ADAR (i.e., ADAR1 and ADAR2) enzymes performs the most common type of RNA editing in metazoans [55], while C-to-U modifications seem to be confined to more discrete transcripts [53, 56–59]. A-to-I RNA editing most frequently targets repetitive RNA sequences located within introns and 5′ and 3′ untranslated regions (UTRs). ADARs use double-stranded RNA as substrates but allow structure interruptions such as bulges and loops. It is well known that these enzymes can use messenger RNA as targets for A-to-I editing and thereby recode the transcript. Both ADAR1 and ADAR2 have been proven to be able to also target short double-stranded RNA molecules, i.e., microRNAs and their precursors. Since the editing activity is found both in the nucleus and the cytoplasm, there are several steps during the microRNA maturation pathway that can be targeted for modification [60]. Although the biological significance of non-coding RNA editing remains largely unknown, several possibilities have been proposed, including its role in the control of endogenous short interfering RNAs [61].
RNA editing involving C-to-U modifications has been reported extensively to play a pivotal role in virus-associated human diseases, including human T lymphotropic virus (HTLV), hepatitis C virus (HCV), hepatitis B virus (HBV), and Epstein-Barr virus (EBV), among others [62, 63]. Furthermore, more recently a possible role in cancer development has also been proposed [63]. However, to date, no abnormalities in C-to-U RNA editing have been reported in cardiovascular diseases.
A-to-I RNA defective editing has been reported in various human diseases including viral infection susceptibility and cancer and neurological and psychiatric disorders [64–68]. Involvement of defective RNA editing in cardiovascular diseases is indirect and scarce [69, 70], yet an involvement in congenital heart diseases is likely to soon emerge.
13.3.2 Pre-mRNA Splicing and Alternative Splicing
RNA splicing is the molecular process by which introns are deleted from nascent immature mRNA providing the means to successfully liked exons back together and thus form a single mature mRNA molecule. RNA splicing is carried out by the assembly of over a hundred core proteins and five small nuclear RNAs into large ribonucleoprotein complexes, named spliceosomes [71]. Regulation of splicing is a complex process [72–74], and alterations of splicing potential have major consequences in distinct human diseases [75].
Alternative splicing is a major driver of protein diversity and allows the generation of distinct proteins from a single gene. It is estimated that almost 85 % of genes within the human genome undergo alternative splicing. Distinct mechanisms such as exon exclusion, intron retention, and the usage of alternative splice sites contribute to modify protein structure, localization, regulation, and function [76, 77]. Interestingly, genetic mutations in distinct spliceosome components have been reported in human families with distinct cardiac diseases such as myocardial infarction [78, 79] and dilated cardiomyopathy [80, 81], suggesting a functional link. Importantly, alternative splicing also plays a pivotal role during embryonic development. Differential expression of distinct spliceosome components has been reported during heart development [82]. Postnatal excitation-contraction coupling impairment has been reported in genetically engineered mice lacking ASF/SF2 spliceosome component [83], and mutant mice for SRp38, a spliceosome regulator, display early embryonic cardiac resulting in impaired calcium handling [84].
On the other hand, alternatively spliced variants have been documented widely in cardiovascular diseases such as cardiomyopathies, arrhythmias, and vascular defects leading to differential expression of sarcomeric proteins, ion channels, and cell signaling proteins [76, 77, 85–89]. An example of the impact of alternative splicing in adult heart physiology is illustrated by the diversity and functional consequences of alternative spliced variants of the troponin-tropomyosin complex (see for a review [90]). Multiple alternatively spliced variants are formed from each of the troponin isoforms, and deregulation of spliced variant expression is linked to dilated cardiomyopathy in different species [91–93]. Similarly, impaired ion channel splice variants also contribute to cardiac arrhythmogenesis, as reported for distinct components of the calcium handling and plasma membrane cardiac pumps [86, 88].
Multiple transcription factors, with critical roles in cardiac development, are alternatively spliced, such as T-box genes [94–96], myocardin [97], myocyte enhancer factor (Mef)-2 [98, 99], pituitary homeobox (Pitx)-2 [100–102], and GATA binding protein 4 (Gata4) [103]. In this context, Yehya et al. [104] identified an intronic retention variant of the NFATC1 (nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1) gene in patients with ventricular septal defects, suggesting that such a spliced variant might be a VSD-susceptibility gene. Bedard et al. [105] reported spliced variants of ZIC3 (Zic family member 3) linked to patients with heterotaxy and congenital heart diseases. McCright et al. [106] reported that aberrant Notch2 alternative spliced variants leads to myocardial hypoplasia as well as eye and kidney defects. More recently, Ricci et al. [107] demonstrate that multiple genes were differentially spliced in hypoplastic left heart syndrome, suggesting a deregulation of cell metabolism and cytoskeleton and cell adherence. Interestingly, impaired alternative splicing in other genes also result in cardiac alterations. Impaired fibronectin splicing is associated with thoracic aortic aneurysm in patients with bicuspid aortic valve [108], while abnormal SCN5A (sodium channel, voltage gated, type V, alpha subunit) alternative spliced variants leads to fetal arrhythmias [109]. Furthermore, impaired expression of alternatively spliced NXT2 (nuclear transport factor 2-like export factor 2) variants, a protein involved in nuclear RNA export, also has been proven to affect cardiac development, particularly valve formation [110]. Ver Heyen et al. [111] reported that genetic engineered disruption of SERCA2a/2b (sarcoplasmic/endoplasmic reticulum calcium ATPase 2a/b) alternative splicing leads to 20 % increase in embryonic and neonatal mortality, as consequence of severe cardiac malformations. Buyon et al. [112] describes a spliced variant of congenital heart block-associated 52 kb autoantigen which is maximal at the time of fetal heart block, suggesting a putative role in its pathophysiology. These reports exemplify the potential causative role of impaired alternative spliced variants as key regulatory modulators of cardiac development. Increasing evidence of this is expected in the coming years as deep-sequencing technologies depict the magnitude of the alternative spliced transcriptome in congenital heart diseases.
13.4 Non-coding RNA-Mediated Posttranscriptional Control
Non-coding RNAs (ncRNAs) constitute a highly diverse group of RNA molecules in structure and function (see for a recent review [113]). Currently ncRNAs are broadly classified according to their size. Small ncRNAs are generally defined as those that are <200 nucleotides, whereas long non-coding RNAs (lncRNAs) can extend to tens or even hundreds of thousands of nucleotides in length. Small ncRNAs display a rather homogeneous structure, whereas lcnRNAs have more complex secondary structures. ncRNAs, such as ribosomal RNAs (rRNAs) and transfer RNAs (tRNAs), have been extensively studied given their prominent roles as components of the translational machinery. A similar situation occurs with small nuclear RNAs (snRNAs) and small nucleolar RNAs (snoRNAs) given their essential role in splicing. Over the last decade, great interest has arisen in a class of small regulatory ncRNAs that directly affect the expression and/or function of protein-coding genes, i.e., microRNAs (miRNAs). miRNAs were discovered in the early 1990s and since then represent the most extensively studied class of ncRNA. microRNAs display an average length of 22–24 nucleotides and are capable of interacting with the 3′ untranslated region of coding RNAs (mRNAs) eliciting blockage of protein translation and/or mRNA degradation [114]. Understanding of microRNA biogenesis has moved rapidly [115], whereas insights into the functional role of microRNAs are progressively emerging at a slower pace. Nonetheless, the functional relevance of distinct microRNAs in multiple aspects of cardiac development and diseases is now widely documented (see for recent reviews [116–118]).
Differential expression of microRNAs has been documented widely during embryonic [119–121], postnatal [122, 123], and the aging heart [124, 125] suggesting a pivotal role for microRNAs during different stages of heart development. Similarly, investigators have reported impaired microRNA expression in a large variety of cardiovascular physiopathological conditions, such as hypertrophic and/or dilated cardiomyopathy [126–131], heart failure [132–134], atrial fibrillation [135–139], and aortic aneurism [140]. The importance of microRNAs in congenital heart diseases is manifested by the embryonic defects observed in genetically engineered mice. Conditional deletion of Dicer, an endonuclease required for the microRNA processing, with distinct Cre drivers, demonstrated the critical role of microRNA biogenesis in distinct temporal and tissue-specific contexts during cardiovascular development. Conditional ablation using an early cardiogenic deletor mouse strain (Nkx2-5-Cre mice) led to embryonic lethality due to cardiac hypoplasia [141], whereas ablation with myocardial-specific Cre driver line (αMHC-Cre) resulted in outflow tract defects and impaired chamber formation [142]. More recently Singh et al. [143] demonstrated that Dicer deletion in pro-epicardial cells compromised cardiac vascular development. In addition, germline deletion of discrete microRNAs such as miR-1-2 resulted in ventricular septal defects and early embryonic lethality [141], whereas miR-126 deletion leads to embryonic lethality due to vascular leakage [144]. These studies highlight the importance of microRNA biology for congenital heart diseases. In this context, an increasing number of studies are providing the impaired microRNA signature of distinct congenital heart diseases [145], such as ventricular septal defects [146], tetralogy of Fallot [147], corrected transposition of great arteries [148], univentricular left hearts [149], bicuspid aortic valves [150], and DiGeorge syndrome [151]. These studies provide novel insights for the prospective use of microRNA signature as biomarkers of prenatal diagnosis [152, 153]. However, in most cases, the impaired regulatory networks modulated by these microRNAs remain to be fully elucidated. In the coming years, we shall see an explosion on the understanding and functional consequences of microRNA regulation, with great hopes as to their therapeutic potential, including pediatric cardiology [154].
In addition to microRNAs, lncRNAs and circular RNAs are emerging also as posttranscriptional modulators. lcnRNAs might undergo alternative splicing and in some cases, but not in others, can be polyadenylated. lncRNAs can be located within the nucleus but also can be found within the cytoplasm thus potentially exerting a large number of biological functions. lncRNAs have been reported in a wide range of functions beyond posttranscriptional regulation such as cell cycle progression, differentiation, apoptosis, structural or cellular trafficking, as well as serving as precursors for smaller RNAs (see for a recent reviews [113, 154–156]). Differential expression of lncRNAs has been reported in the developing [157–159], adult [160] and aging [161] heart as well as in ventricular cardiac hypertrophy [161], heart failure [134], myocardial infarction [162], and cardiac ischemia [163]. Interestingly, a pivotal role of myheart lcnRNA has been reported in the context of cardiac hypertrophy [164, 165]. Importantly, differentially expression of lcnRNAs also has been reported in hearts with congenital heart defects, such as ventricular septal defect [166] and tetralogy of Fallot [167]. Overall these data suggest a plausible role for lncRNAs in congenital heart diseases, and the first evidences for this have recently been reported. Seminal works demonstrated that genetic deletion of fendrr and braveheart, two cardiac enriched lncRNAs, respectively, leads to impaired cardiogenesis [168, 169]. On the other hand, understanding of the functional role of circular RNAs is very incipient, with yet some evidence that they can act as microRNA sponges [170, 171]. In the coming years, it is expected that unraveling the functional roles of lcnRNAs and circular RNAs will guide toward the understanding of the etiology of distinct cardiovascular diseases, including there in congenital heart diseases.
Conclusion
Posttranscriptional regulation is a complex process. This chapter has highlighted distinct processes that sequentially modify the nascent mRNA molecule into a mature form with, in many cases multiple distinct variants. It is important to emphasize that complex regulatory networks between these processes are well documented such as for the multiple roles of 5′ capping and 3′ polyadenylation in mRNA stabilization, elongation, and translation among others, but importantly emerging evidence demonstrates that microRNAs and lncRNAs also participate in these intricately interlinked regulatory mechanisms [172], i.e., modulating alternative splicing [173]. Thus, we could foresee that in coming years, impaired posttranscriptional regulatory networks would be linked to distinct congenital heart diseases, as recently reported by Xu et al. [174].
References
1.
Lukong KE, Chang KW, Khandjian EW et al (2008) RNA-binding proteins in human genetic disease. Trends Genet 24:416–425PubMed
2.
Blech-Hermoni Y, Ladd AN (2013) RNA binding proteins in the regulation of heart development. Int J Biochem Cell Biol 45:2467–2478PubMedCentralPubMed
3.
Forget A, Chartrand P (2011) Cotranscriptional assembly of mRNP complexes that determine the cytoplasmic fate of mRNA. Transcription 2:86–90PubMedCentralPubMed
4.
Fresco LD, Buratowski S (1996) Conditional mutants of the yeast mRNA capping enzyme show that the cap enhances, but is not required for, mRNA splicing. RNA 2:584–596PubMedCentralPubMed
5.
Shatkin AJ, Manley JL (2000) The ends of the affair: capping and polyadenylation. Nat Struct Biol 7:838–842PubMed
6.
Glover-Cutter K, Kim S, Espinosa J et al (2008) RNA polymerase II pauses and associates with pre-mRNA processing factors at both ends of genes. Nat Struct Mol Biol 15:71–78PubMedCentralPubMed
8.
Suh MH, Meyer PA, Gu M et al (2010) A dual interface determines the recognition of RNA polymerase II by RNA capping enzyme. J Biol Chem 285:34027–34038PubMedCentralPubMed
9.
Yue Z, Maldonado E, Pillutla R et al (1997) Mammalian capping enzyme complements mutant Saccharomyces cerevisiae lacking mRNA guanylyltransferase and selectively binds the elongating form of RNA polymerase II. Proc Natl Acad Sci U S A 94:12898–12903PubMedCentralPubMed
10.
Tsukamoto T, Shibagaki Y, Niikura Y et al (1998) Cloning and characterization of three human cDNAs encoding mRNA (guanine-7-)-methyltransferase, an mRNA cap methylase. Biochem Biophys Res Commun 251:27–34PubMed
11.
Yamada-Okabe T, Doi R, Shimmi O et al (1998) Isolation and characterization of a human cDNA for mRNA 5′-capping enzyme. Nucleic Acids Res 26:1700–1706PubMedCentralPubMed
12.
Pillutla RC, Shimamoto A, Furuichi Y et al (1998) Human mRNA capping enzyme (RNGTT) and cap methyltransferase (RNMT) map to 6q16 and 18p11.22-p11.23, respectively. Genomics 1998(54):351–353
13.
Ishikawa K, Nagase T, Nakajima D et al (1997) Prediction of the coding sequences of unidentified human genes. VIII. 78 new cDNA clones from brain which code for large proteins in vitro. DNA Res 4:307–313PubMed
14.
Konarska MM, Padgett RA, Sharp PA (1984) Recognition of cap structure in splicing in vitro of mRNA precursors. Cell 38:731–736PubMed
15.
Spriggs KA, Stoneley M, Bushell M et al (2008) Re-programming of translation following cell stress allows IRES-mediated translation to predominate. Biol Cell 100:27–38PubMed
16.
Furuichi Y, LaFiandra A, Shatkin AJ (1977) 5′-Terminal structure and mRNA stability. Nature 266:235–239PubMed
17.
Shimotohno K, Kodama Y, Hashimoto J et al (1977) Importance of 5′-terminal blocking structure to stabilize mRNA in eukaryotic protein synthesis. Proc Natl Acad Sci U S A 74:2734–2738PubMedCentralPubMed
18.
Murthy KG, Park P, Manley JL (1991) A nuclear micrococcal-sensitive, ATP-dependent exoribonuclease degrades uncapped but not capped RNA substrates. Nucleic Acids Res 19:2685–2692PubMedCentralPubMed
19.
Schwer B, Shuman S (1996) Conditional inactivation of mRNA capping enzyme affects yeast pre-mRNA splicing in vivo. RNA 2:574–583PubMedCentralPubMed
20.
Schwer B, Mao X, Shuman S (1998) Accelerated mRNA decay in conditional mutants of yeast mRNA capping enzyme. Nucleic Acids Res 26:2050–2057PubMedCentralPubMed
21.
Flaherty SM, Fortes P, Izaurralde E et al (1997) Participation of the nuclear cap binding complex in pre-mRNA 3′ processing. Proc Natl Acad Sci U S A 94:11893–11898PubMedCentralPubMed
22.
Curinha A, Braz SO, Pereira-Castro I et al (2014) Implications of polyadenylation in health and disease. Nucleus 5:508–519PubMedCentralPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


