Post-Myocardial Infarction Risk Stratification and Management
Willis M. Wu
Venu Menon
I. INTRODUCTION.
More than 1.5 million patients will have an acute coronary syndrome (ACS) in the United States each year. At least 1 million will have evidence of myocardial infarction (MI), for which mortality and morbidity remain considerable. Although patient outcomes have improved, well-documented therapies are still often underprescribed. Besides identifying patients with MI, the goals of the physician must be to successfully stratify patients according to risk, implement medical interventions, and initiate risk factor modification during the initial hospitalization.
II. RISK STRATIFICATION.
Post-MI risk stratification identifies patients at high risk for subsequent cardiovascular events who will benefit from revascularization.
A. Age
is the most important predictor of mortality after MI. The average age of patients with first MI is approximately 65 years. Although older patients are at greatest risk and may benefit most, they receive less aggressive treatment compared with younger patients, who have the lowest overall mortality.
B. Assessment of left ventricular (LV) function
1. LV function is the second most important predictor of mortality after MI. An inverse relation exists between left ventricular ejection fraction (LVEF) and mortality. Mortality is greatest for patients with an LVEF <40%.
2. Assessment of LV function is indicated for all patients diagnosed with MI. Echocardiography is often utilized to assess LV function because it is readily available, is relatively inexpensive, and can assess concomitant valvular function as well as mechanical complications of MI. Left ventriculography performed during diagnostic catheterization, or ascertained by radionuclide ventriculography, and cardiac magnetic resonance may also be utilized to evaluate LV function. Availability, local expertise, and cost are important considerations when deciding which procedure to use.
C. Other indicators.
Biomarkers are useful in further risk-stratifying patients after MI. Cardiac troponin elevation identifies high-risk patients and incremental increases in troponin levels predict higher mortality in patients with ACS. Elevated serum levels of high-sensitivity C-reactive protein and B-type natriuretic peptide may also provide prognostic information. New ST-segment changes, both elevation and depression, portend higher risk of death, heart failure, recurrent ischemia, and severe coronary artery disease (CAD). Electrical instability, such as ventricular arrhythmias and atrial fibrillation, are associated with increased risk. Anterior MI, renal insufficiency, poor glycemic control, and anemia are also associated with worse outcomes.
D. Risk models.
Various models utilize a combination of the aforementioned risk factors to quantitate a predictive score of patient risk for subsequent cardiac events
and mortality. Examples include Thrombolysis in Myocardial Infarction (TIMI), Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardio (GISSI), and the Global Registry of Acute Coronary Events (GRACE).
and mortality. Examples include Thrombolysis in Myocardial Infarction (TIMI), Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardio (GISSI), and the Global Registry of Acute Coronary Events (GRACE).
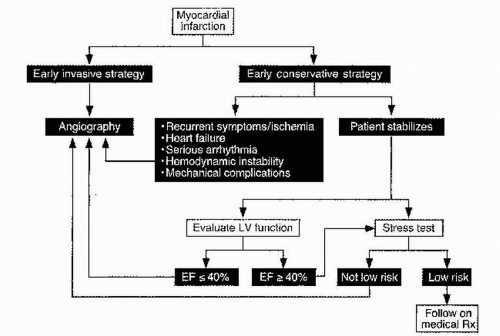
E. Assessment of residual ischemia
(Fig. 5.1). The extent of CAD and the presence of residual ischemia are two strong predictors of mortality among patients who have had an MI. For this reason, post-MI patients who have not undergone angiography should undergo stress testing before discharge or shortly thereafter (preferably within 3 to 7 days post-MI). Non-ST-elevation ACS patients at low or intermediate risk who do not have ongoing ischemia or heart failure for at least 12 to 24 hours are also candidates for submaximal stress testing. Because of increasing availability of percutaneous revascularization and growing implementation of a pharmaco-invasive strategy for ST-elevation MI (STEMI) patients who receive thrombolytics, most patients with STEMI undergo angiographic definition. Stable STEMI patients who have not undergone catheterization should undergo stress testing to assess for ischemia 2 to 3 days after the index event.
1. Submaximal exercise stress testing is optimal for noninvasive risk stratification. This test provides considerable prognostic information, assesses functional capacity and efficacy of medical therapy, and can guide cardiac rehabilitation after discharge. Patients who achieve at least 3 metabolic equivalents (METs) of the task have a good prognosis. Inability to achieve 3 METs, hypotension during exercise, or marked ST-segment depression or elevation is an indication for coronary angiography. 2. Stress imaging with echocardiography or radionuclide imaging is recommended in patients who have uninterpretable electrocardiograms (e.g., baseline ST-T changes, LV hypertrophy, intraventricular conduction delays, paced rhythm, or digoxin-related effects). Addition of either imaging modality increases both the sensitivity and specificity of detecting CAD. Patients with
severe resting or exercise-induced LV dysfunction or evidence of extensive ischemia (large perfusion defect, multiple moderate perfusion defects, wall motion abnormalities at low-dose dobutamine or low heart rate, and stress-induced LV dilation) are considered high risk and should undergo coronary angiography.
severe resting or exercise-induced LV dysfunction or evidence of extensive ischemia (large perfusion defect, multiple moderate perfusion defects, wall motion abnormalities at low-dose dobutamine or low heart rate, and stress-induced LV dilation) are considered high risk and should undergo coronary angiography.
3. Dobutamine, adenosine, and dipyridamole are pharmacologic agents used safely in conjunction with imaging for post-MI stress testing if a patient cannot exercise (see Chapter 6).
III. THERAPY AFTER MI
A. Coronary angiography
1. Indications
a. Coronary angiography is indicated in patients with STEMI as well as in those with non-STEMI who are at high risk for clinical events, recurrent angina or ischemia on medical therapy, or high-risk findings on noninvasive stress testing.
b. An “early invasive strategy” utilizing coronary angiography is the preferred approach for patients at high risk for clinical events, including a high-risk score, elevated troponin, congestive heart failure, mechanical complications, and electrical or hemodynamic instability.
c. Patients with a history of prior revascularization, either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), should generally be referred for angiography.
d. Coronary angiography to identify potential bypass targets is generally preferred prior to open heart surgery if a patient has surgical anatomy or has a mechanical complication post-MI requiring surgical intervention. Such complications of MI include ventricular septal rupture, LV aneurysm, and acute mitral regurgitation due to papillary muscle rupture. In rare instances when hemodynamic instability precludes angiography, the surgeon may bypass all or selected coronary arteries.
2. Contraindications.
Catheterization should not be performed on patients who are ineligible for surgical or percutaneous revascularization because of severe comorbid conditions or who do not consent to angiography because of personal preference.
3. Controversy.
Low-risk, asymptomatic patients who have sustained an uncomplicated MI generally have a good long-term prognosis and may not need to undergo angiography. These patients presumably are those without high-risk features on noninvasive stress testing who will receive aggressive medical therapy and risk factor modification.
4. PCI.
A new era of PCI therapy that includes novel anticoagulants, antiplatelet agents, and newer generation drug-eluting stents continues to significantly enhance the options and outcomes for post-MI patients undergoing revascularization.
B. CABG after MI
can be divided into two categories: emergent and elective.
1. Emergent CABG.
The indications and management considerations for emergent CABG are discussed in Chapter 1.
2. Elective CABG.
CABG has been shown to provide a survival benefit for patients with left main (>50% stenosis) or extensive three-vessel CAD. Surgical revascularization remains preferable for patients with severe LV dysfunction, diabetes, or two-vessel disease with proximal left anterior descending involvement and either high-risk noninvasive stress test results or LV dysfunction. However, the Arterial Revascularization Therapies Study (ARTS) demonstrated no significant difference in mortality, MI, or stroke among patients with multivessel disease and normal to moderately decreased LV function randomized to CABG versus PCI. The Synergy between Percutaneous Coronary Intervention with Taxus and
Cardiac Surgery (SYNTAX) trial evaluated PCI versus CABG for three-vessel or left main disease and nearly 30% of the patients had ACS. This trial demonstrated equivalent cumulative rates of major adverse cardiac or cerebrovascular events in the low (0 to 22) and intermediate (22-32) SYNTAX score patients, though there was a higher rate of adverse events in the high (≥33) SYNTAX score patients who underwent PCI. Target vessel revascularization was higher in the PCI group but stroke rate was higher in the CABG group.
Cardiac Surgery (SYNTAX) trial evaluated PCI versus CABG for three-vessel or left main disease and nearly 30% of the patients had ACS. This trial demonstrated equivalent cumulative rates of major adverse cardiac or cerebrovascular events in the low (0 to 22) and intermediate (22-32) SYNTAX score patients, though there was a higher rate of adverse events in the high (≥33) SYNTAX score patients who underwent PCI. Target vessel revascularization was higher in the PCI group but stroke rate was higher in the CABG group.
3. Operative risk.
No prospective, randomized trials have been performed to determine the optimal timing of elective CABG after MI. Most data suggest that CABG 3 to 7 days after MI is associated with a low operative mortality similar to that of elective bypass in patients without recent infarction. Operative risk increases among patients with LV dysfunction, advanced age, and multiple comorbid conditions (e.g., diabetes mellitus, chronic obstructive pulmonary disease, and chronic renal insufficiency). Emergent CABG and reoperations on patients with prior open heart surgery are associated with a higher operative mortality. If initiated previously, clopidogrel should be held for at least 5 days prior to surgery and prasugrel for at least 7 days prior to decrease perioperative bleeding risk. Ticagrelor is a reversible inhibitor of the adenosine diphosphate (ADP) receptor P2Y12 that is more rapid acting and potent than clopidogrel. There was initial hope that the use of ticagrelor in patients with ACS undergoing urgent bypass surgery could reduce the time from cessation of antiplatelet agents to the time of surgery to just 2 or 3 days, given the more rapid reversal of platelet inhibition. However, results from the Study of Platelet Inhibition and Patient Outcomes (PLATO) demonstrated no difference in TIMI major bleeding or transfusion rates in patients with ACS who stopped clopidogrel 5 days prior to CABG compared with those who stopped ticagrelor 24 to 72 hours before surgery. Bleeding rates were equivalent even when surgery occurred 1 to 3 days after discontinuation of antiplatelets. Therefore, current ACC/AHA guidelines recommend withholding ticagrelor for at least 5 days prior to elective open heart surgery.
IV. SECONDARY PREVENTION
A. Smoking cessation is mandatory.
Smoking doubles the rate of reinfarction and death after MI, causes coronary artery spasm, and reduces the effectiveness of β-blocker therapy. The risk reduction attributed to smoking cessation is rapid and nearly equals that of post-MI patients who never smoked in only 3 years. Half of all patients who stop smoking after MI will begin smoking again within 6 to 12 months. Many approaches to smoking cessation have been attempted, including pharmacologic therapy, formal smoking cessation programs, hypnosis, and abstinence.
1. Nicotine substitutes can be delivered by a variety of vehicles, including transdermal patches, chewing gum, nasal spray, and inhalers. These systems can deliver 30% to 60% of the nicotine of cigarettes. Although nicotine substitutes are not recommended for the acute phase of MI, use of these agents is safe in later phases. Patients who start smoking again should discontinue the use of nicotine substitutes.
2. Pharmacotherapy. Bupropion appears to be an effective aid in smoking cessation. The dose is doubled after 3 days and it is then taken twice daily for 7 to 12 weeks. Patients set a goal to stop smoking 1 to 2 weeks into therapy. Varenicline, a partial agonist of nicotine receptors, provides nicotine stimulation while blocking cigarette nicotine effects. In a head-to-head trial, varenicline was more effective than bupropion at the 12-week time period, but data suggest no significant difference in rates of abstinence at 1 year. In addition, the FDA issued a communication in 2011 warning that varenicline may increase the risk of cardiovascular events.
3. Recommendations. Physicians can aid patients in their effort to stop smoking by using a stepped approach with education and a firm recommendation to quit smoking, devising a plan, and reinforcing the need to quit. Patients who are likely to relapse are older, less educated, or heavy smokers. Formal smoking cessation programs have been shown to have high rates of patient abstinence. Coinhabitants should also stop smoking to increase the likelihood of success.
B. Lipid management
1. Low-density lipoprotein (LDL).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree