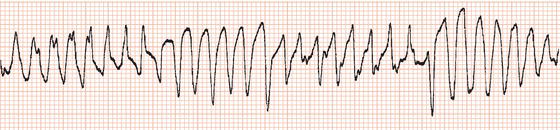
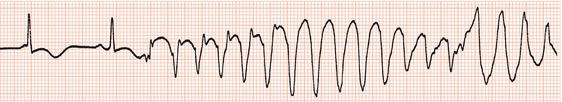
and Ventricular Fibrillation
‘Torsade de pointes’ tachycardia refers to polymorphic tachycardia when there is QT prolongation preceding the arrhythmia: correction of or treatment directed at the cause of QT prolongation is required, rather than antiarrhythmics. Causes of torsade de pointes include bradycardia; drugs (e.g. erythromycin and the antipsychotics) which prolong the QT interval; and the hereditary long QT syndromes. The hereditary long QT syndromes can cause syncope and sudden death: treatment is with beta-blockers and/or an implantable defibrillator.
The Brugada syndrome is a genetic disorder characterised by downsloping ST elevation in the right precordial leads, and it may cause ventricular fibrillation. Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare, genetically determined condition, in which polymorphic or bidirectional ventricular tachycardia can be induced by exercise, requiring beta-blockers and/or an implantable defibrillator.
Polymorphic ventricular tachycardia
Whereas monomorphic ventricular tachycardia consists of a rapid succession of ventricular ectopic beats each with the same configuration, polymorphic tachycardia is characterised by repeated progressive changes in the direction and amplitude of ventricular complexes so that they appear to ‘twist’ about the baseline (Figure 13.1). It may result from myocardial infarction or from cardiomyopathy. In these situations the QT interval during sinus rhythm is normal, and the management of the arrhythmia is the same as for monomorphic ventricular tachycardia.
QT interval
The QT interval is a measure of the duration of the process of ventricular depolarisation and subsequent repolarisation. It is measured from the onset of the QRS complex to the end of the T wave.
Normally the QT interval shortens with increasing heart rate, partly due to the increase in rate itself and partly due to the increase in sympathetic nervous system activity which causes sinus tachycardia. When measuring the QT interval it is therefore necessary to correct the measured interval for heart rate. The corrected QT interval (QTc) is calculated by selecting the ECG lead showing the longest QT interval (usually measurements are made from lead II and V5), and then dividing the square root of the cycle length into the measured QT interval (Bazett’s formula). For example, a patient with a measured QT interval of 0.38 s at a heart rate of 60 beats/min has a cycle length of 1.0 s and therefore also has a QTc of 0.38 s, whereas with a QT interval of 0.38 s at a heart rate of 120 beats/min (cycle length = 0.5 s), the QTc is prolonged at 0.54 s. The normal QTc does not exceed 0.43 s in males and 0.45 s in females.
Always consider QT prolongation if the end of the T wave approaches the midpoint between QRS complexes.
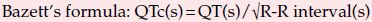
Causes of QT prolongation include the hereditary long QT syndromes, hypocalcaemia, hypothyroidism, a number of drugs, myocardial ischaemia and subarachnoid haemorrhage.
Torsade de pointes tachycardia
‘Torsade de pointes’ is the term applied to polymorphic ventricular tachycardia when the QT interval is prolonged preceding the onset of the arrhythmia (Figures 13.2, 13.3). Recognition is important, because correction of or treatment directed at the QT prolongation is required rather than antiarrhythmic drugs, which may aggravate the tachycardia. Typically the arrhythmia is non-sustained and repetitive, but it can deteriorate to ventricular fibrillation. Onset usually follows a pause in rhythm caused by bradycardia or following an ectopic beat.
Figure 13.3 Two brief episodes of torsade de pointes tachycardia during sinus bradycardia; there is marked QT prolongation preceding the arrhythmia.
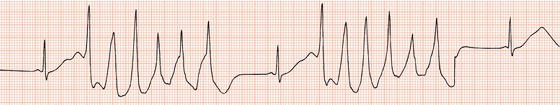
Bradycardia due to sinus node dysfunction or atrioventricular block
Hypocalcaemia, hypomagnesaemia, hypothyroidism
Antiarrhythmic drugs, e.g. quinidine, disopyramide, procainamide, sotalol, amiodarone, ibutilide, dofetilide, dronedarone
Antimicrobials, e.g. erythromycin, clarithromycin, ciprofloxacin, fluconazole, ketoconazole, chloroquine, pentamidine
Psychiatric drugs, e.g. thioridazine, chlorpromazine, pimozide, haloperidol, droperidol, tricyclic antidepressants, methadone, citaloprolam
Other drugs: prenylamine, bepridil, cisapride, terfenadine, probucol, domperidone, tamoxifen, ondansetron
Anorexia nervosa
Figure 13.4 Complete AV block leading to (a) a very long QT interval, with (b) episodes of torsade de pointes tachycardia.
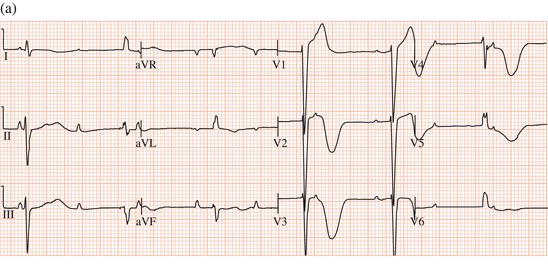
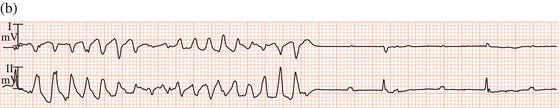
Torsade de pointes tachycardia can be caused by disorders that lead to abnormal ventricular repolarisation, by bradycardia (Figure 13.4), or by drugs.
Antipsychotic drugs
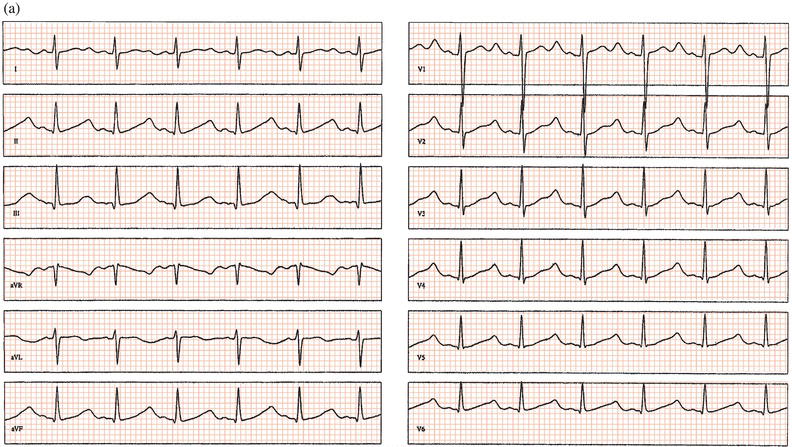
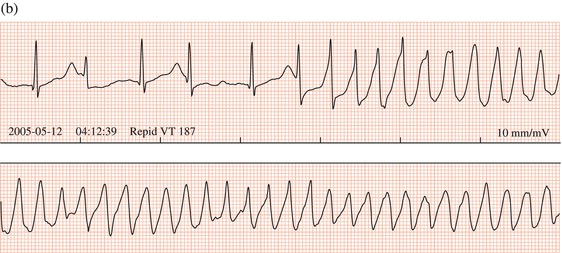
Antipsychotic drugs are an important cause of QT prolongation and torsade de pointes tachycardia (Figure 13.5). In a large study, chlorpromazine, haloperidol, pimozide, and thioridazine were shown to increase the risk of sudden cardiac death threefold; female patients and those who had recently started medication were at highest risk. It is recommended that the QT interval be monitored by carrying out a baseline ECG before antipsychotic therapy and repeating it after a few days and then every 1–3 months in the early stages of high-dose treatment. Recently, citaloprolam and escitaloprolam have been associated with a dose-dependent QT prolongation: they should be avoided if there is known QT prolongation or hereditary long QT syndrome, or if other drugs that can prolong the QT interval are being prescribed.
Figure 13.5 Overdose of the antipsychotic amisulpride leading to (a) QT prolongation and (b) torsade de pointes tachycardia. T wave alternans precedes the arrhythmia.
Drug interactions
Risk of torsade de pointes may be increased if an interaction between drugs leads to a higher blood level of a potentially proarrhythmic drug. For example, the erythromycins are metabolised by the liver’s cytochrome P450 3A enzyme system. Commonly used drugs such as diltiazem and verapamil, as well as a number of antifungal agents, inhibit the enzyme system and have been shown to increase the risk of sudden death when administered together with erythromycin or clarithromycin.
Management of torsade de pointes tachycardia
Treatment consists of reversal of the cause where possible, and cardiac pacing. Intravenous magnesium sulphate may be effective, even when serum magnesium is normal (8 mmol stat; 2.5 mmol/hour infusion). Antiarrhythmic drugs should be stopped: increasing the heart rate to 100 beats/min by pacing will often prevent the tachycardia while the drug(s) are being excreted or metabolised.
Hereditary long QT syndromes
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree