Pneumothorax
A pneumothorax is air in the pleural space, that is, air between the lung and the chest wall. Pneumothoraces can be divided into spontaneous pneumothoraces, which occur without antecedent trauma or other obvious cause, and traumatic pneumothoraces, which occur from direct or indirect trauma to the chest. A subcat-egory of traumatic pneumothorax is iatrogenic pneumothorax, which occurs as an intended or inadvertent consequence of a diagnostic or therapeutic maneuver. Spontaneous pneumothoraces are further divided into primary and secondary spontaneous pneumothoraces. Primary spontaneous pneumothoraces occur in otherwise healthy individuals, whereas secondary spontaneous pneumothoraces occur as a complication of underlying lung disease, most commonly chronic obstructive pulmonary disease (COPD).
PRIMARY SPONTANEOUS PNEUMOTHORAX
Incidence
The most complete figures on the incidence of primary spontaneous pneumothorax probably come from a study of the residents of Olmsted County, Minnesota, where complete medical records are kept on all residents. Between 1959 and 1978, 77 cases of primary pneumothorax occurred among the county’s population that averaged 60,000 over this period. The age-adjusted incidence of primary spontaneous pneumothorax was 7.4/100,000/year for men and 1.2/100,000/year for women (1). If these figures are extrapolated to the entire population of 250 million in the United States, one can anticipate approximately 10,000 new cases of primary spontaneous pneumothorax per annum. In a more recent study from the United Kingdom, the incidence of spontaneous pneumothorax in males and females aged 15 to 34 was 37.0 and 15.4/100,000/year, respectively. Since most patients in this age range have primary spontaneous pneumothorax, it appears that the incidence in the United Kingdom is greater than that previously reported in the United States (2).
Etiologic Factors
The general consensus is that primary spontaneous pneumothorax results from rupture of subpleural emphysematous blebs that are usually located in the apices of the lung (3,4). In one older study, Gobbel et al. operated on 31 patients with primary spontaneous pneumothorax and found subpleural blebs or bullae in each patient (3). In a more recent study, Lesur et al. (4) obtained computed tomography (CT) scans on 20 young (mean age 27) patients with spontaneous pneumothorax and could demonstrate apical subpleural emphysematous lesions in 16 of the 20 patients (80%). In another study, Bense et al. obtained CT scans on 27 nonsmoking patients with primary spontaneous pneumothorax and reported that 22 (81%) had emphysema-like changes, mostly in the upper lobes (5). It appears that the apical blebs present on direct visualization and the emphysema-like changes seen on CT scan represent the same abnormality. It should be noted, however, that there is some controversy concerning the significance of the subpleural blebs. Noppen et al. have identified abnormal regions of the visceral pleura by fluoresceinenhanced autofluorescence thoracoscopy and suggest that leakage of air through these areas, rather than rupture of blebs, may be responsible for primary spontaneous pneumothorax (6). I believe that most primary spontaneous pneumothoraces are due to
rupture of a bleb. The reason that I believe this is that the symptoms of primary spontaneous pneumothorax start suddenly. If the pneumothorax were due to slow leakage through the visceral pleura, the symptoms should not start abruptly.
rupture of a bleb. The reason that I believe this is that the symptoms of primary spontaneous pneumothorax start suddenly. If the pneumothorax were due to slow leakage through the visceral pleura, the symptoms should not start abruptly.
The pathogenesis of these subpleural blebs is probably related to airway inflammation. Respiratory bronchiolitis was found in 70 of 79 patients (89%) who underwent a surgical procedure for recurrence or persistence of primary spontaneous pneumothorax (7). All the patients in this study were smokers, and cigarette smoking can certainly produce airway inflammation. Cigarette smoking is known to be strongly associated with the development of primary spontaneous pneumothorax. When the smoking habits of 505 patients from four separate studies were analyzed (8,9,10,11), 461 of the patients (91%) were smokers. Furthermore, the occurrence of a spontaneous pneumothorax appears to be related to the level of cigarette smoking. Compared with nonsmokers, the relative risk of a pneumothorax in men is seven times higher in light smokers (1-12 cigarettes/day), 21 times higher in moderate smokers (13-22 cigarettes/ day), and 102 times higher in heavy smokers (>22 cigarettes/day). For women, the relative risk is 4, 14, and 68 times higher in light, moderate, and heavy smokers than in nonsmokers, respectively (11). Disease of the small airways related to smoking probably contributes to the development of the subpleural blebs (12). Interestingly, the prevalence of smoking in Chinese patients with primary spontaneous pneumothorax is only about 50% (13,14).
Two studies concluded that spontaneous pneumothoraces were more likely to develop following days when there are broad swings in the atmospheric pressure (15,16). It was postulated that the air in the apical blebs was not in free communication with the airways. Therefore, when the atmospheric pressure falls, the distending pressure of the bleb may increase and could result in its rupture (15). It should be noted that three other studies found no relationship between change in the atmospheric pressure and the occurrence of a spontaneous pneumothorax (17,18,19). In one study, however, there was a significant relationship between thunderstorms and the occurrence of pneumothoraces (17). Noppen et al. described the development of five episodes of primary spontaneous pneumothorax in four patients upon exposure to loud music (20).
Patients with primary spontaneous pneumothorax are usually taller and thinner than control patients. In a study of military recruits with pneumothorax, Withers et al. (21) found that those with pneumothoraces were 2 in. taller and 25 lb lighter than the average military recruit (21). Because the gradient in pleural pressure is greater from the lung base to the lung apex in taller individuals (see Chapter 2), the alveoli at the lung apex are subjected to a greater mean distending pressure in taller individuals. Over a long period, this higher distending pressure could lead to the formation of subpleural blebs in taller individuals who are genetically predisposed to bleb formation.
The tendency to develop a spontaneous pneumothorax may be genetically determined (22). There is a high incidence of pneumothorax in patients with the Birt-Hogg-Dubé syndrome. This syndrome is autosomal dominant (23) and is characterized by an increased incidence of spontaneous pneumothorax, benign skin tumors, and renal tumors (23,24). The gene has been mapped to chromosome 17p11.2 (23). Mutations in the folliculin (FLCN) gene are responsible for the Birt-Hogg-Dubé syndrome (25). At least 53 different germline mutations and 31 SNPs have been identified in patients with the Burt-Hogg-Dubé syndrome (26). Pneumothorax occurred in 25 of 111 patients (22.5%) in one study (27). Radiographically, 15% to 83% have pulmonary cysts and/or bullae (28). When these patients are explored surgically, they are found to have apical blebs (27). Microscopic examination of the resected lung tissue reveals cysts comprising intraparenchymal collections of air surrounded by normal parenchyma or a thin fibrous wall or blebs consisting of collections of air within the pleura (28).
There have been other reports of a familial tendency for the development of primary spontaneous pneumothorax. In one study of primary spontaneous pneumothorax in the Israeli Defense Forces, 11.5% of 286 patients with spontaneous pneumothorax had a positive family history for primary spontaneous pneumothorax (29). A more in-depth analysis of 15 families suggested that the mode of inheritance for the tendency for pneumothorax was either autosomal dominant with incomplete penetrance or X-linked recessive (30). These reports were written before the Birt-Hogg-Dubé syndrome was described and the patients may have had this syndrome. In another report of patients with familial pneumothorax, individuals with human leukocyte antigen (HLA) haplotype A2, B40 were found to be much more likely to have a pneumothorax (31). Other studies of familial pneumothorax have been unable to document any association with the HLA haplotypes (32).
Well-known inherited diseases associated with pneumothorax include Marfan’s syndrome, homocystinuria, Ehlers-Danlos syndrome, and α1-antitrypsin deficiency (24).
Well-known inherited diseases associated with pneumothorax include Marfan’s syndrome, homocystinuria, Ehlers-Danlos syndrome, and α1-antitrypsin deficiency (24).
There is a very high prevalence of bronchial abnormalities in nonsmoking patients with spontaneous pneumothorax. Bense et al. (33) performed fiberoptic bronchoscopy on 26 people who had never smoked but had a history of spontaneous pneumothorax. They reported that 25 of 26 (96%) of the patients had bronchial abnormalities bilaterally. In comparison, only 1 of 41 control patients had such abnormalities (33). The bronchial abnormalities included disproportionate bronchial anatomy (smaller than normal dimensions and deviating anatomic arrangements of the airways at various locations), an accessory bronchus, or a missing bronchus. The most common abnormality was the disproportionate bronchial anatomy (33).
Pathophysiologic Features
When resected specimens from the lungs of patients with spontaneous pneumothorax are examined, there is frequently an eosinophilic pleuritis (34). In addition, some patients have mild pulmonary vascular and perivascular eosinophilia (34). Many patients also have pulmonary artery intimal fibrosis and pulmonary vein intimal fibrosis (35). The eosinophilic pleuritis is probably directly related to the air in the pleural space, and the presence of abnormalities in the pulmonary vessels should not serve as an indication to investigate the patient for pulmonary vascular disease.
The physiological consequences of a pneumothorax are discussed in Chapter 3.
Clinical Manifestations
The peak age for the occurrence of a primary spontaneous pneumothorax is the early 20s, and primary spontaneous pneumothorax rarely occurs after age 40. The main symptoms associated with the development of primary spontaneous pneumothorax are chest pain and dyspnea. In a series of 39 patients reported by Vail et al. (36), every patient had chest pain or dyspnea, and both symptoms were present in 25 of the 39 patients (64%). Seremetis (10) reported chest pain in 140 of 155 patients (90%). The chest pain usually has an acute onset and is localized to the side of the pneumothorax. On rare occasions, the patient has neither chest pain nor dyspnea. The pneumothorax is bilateral in less than 2% of patients (13). In the series of Seremetis (10), five patients (3%) complained only of generalized malaise. On rare occasions, the pneumothorax is discovered on a routine chest radiograph (37). Horner’s syndrome has been reported as a rare complication of spontaneous pneumothorax and is thought to be due to traction on the sympathetic ganglion produced by shift of the mediastinum (38).
Primary spontaneous pneumothorax usually develops while the patient is at rest. In the series of 219 patients of Bense et al. (39), 87% were at rest at the onset of symptoms and none were exerting themselves heavily when symptoms began. Other series have reported comparable findings (8,10).
Many patients with spontaneous pneumothorax do not seek medical attention immediately after the development of the symptoms. Eighteen percent of the patients in one series had symptoms for more than a week before seeking medical attention (10), whereas 46% in a second series waited more than 2 days before seeing a physician (8). Patients with symptoms for more than 3 days should not have negative pressure applied to their chest tubes in view of the higher incidence of reexpansion pulmonary edema with prolonged pneumothorax (see Chapter 28).
Changes on Physical Examination
Physical examination of patients with primary spontaneous pneumothorax reveals vital signs that are usually normal, with the exception of a moderate tachycardia. If the pulse rate exceeds 140 or if hypotension, cyanosis, or electromechanical dissociation is present, a tension pneumothorax should be suspected (see the section later in this chapter on tension pneumothorax). Examination of the chest reveals that the side with pneumothorax is larger than the contralateral side and moves less during the respiratory cycle. Tactile fremitus is absent, the percussion note is hyperresonant, and the breath sounds are absent or reduced on the affected side. The trachea may be shifted toward the contralateral side. With right-sided pneumothoraces, the lower edge of the liver may be shifted inferiorly.
Electrocardiographic Changes
Patients with spontaneous pneumothorax may show electrocardiographic changes due to the pneumothorax (40). In a study of seven patients with spontaneous left pneumothorax, Walston et al. (41) found that a rightward shift of the frontal QRS axis, a diminution of precordial R voltage, a decrease in QRS amplitude,
and precordial T-wave inversion could all occur. A different study (40) reports that the V2-6 amplitude was decreased with left-sided pneumothorax. In a study (42) of patients with right-sided pneumothorax, prominent R-wave voltage in lead V2 with loss of S-wave voltage, mimicking posterior myocardial infarction, and reversible reduced QRS voltage were reported. In another study (40), the QRS amplitude was increased in V5-6 with right-sided pneumothorax (40). In addition, marked PR-segment elevation in the inferior leads with reciprocal PR-segment depression in aVR has been reported (43). These changes should not be mistaken for an acute non-Q wave myocardial infarction. There has also been a report of a patient with a tension pneumothorax who developed pronounced ST-segment elevation in II, III, a VF, and V4-6 (44). When a chest tube was inserted, the ST changes resolved and studies of myocardial enzymes were negative (44).
and precordial T-wave inversion could all occur. A different study (40) reports that the V2-6 amplitude was decreased with left-sided pneumothorax. In a study (42) of patients with right-sided pneumothorax, prominent R-wave voltage in lead V2 with loss of S-wave voltage, mimicking posterior myocardial infarction, and reversible reduced QRS voltage were reported. In another study (40), the QRS amplitude was increased in V5-6 with right-sided pneumothorax (40). In addition, marked PR-segment elevation in the inferior leads with reciprocal PR-segment depression in aVR has been reported (43). These changes should not be mistaken for an acute non-Q wave myocardial infarction. There has also been a report of a patient with a tension pneumothorax who developed pronounced ST-segment elevation in II, III, a VF, and V4-6 (44). When a chest tube was inserted, the ST changes resolved and studies of myocardial enzymes were negative (44).
Diagnosis
In a young, thin, tall man, the diagnosis is usually suggested by the clinical history and physical examination. The diagnosis is established by demonstrating a pleural line on the chest radiograph (Fig. 6.12) (45). Because expiratory chest radiographs have little or no advantage over inspiratory chest radiographs in making the diagnosis of pneumothorax (46), they are not routinely recommended. However, if there is a strong suspicion of a pneumothorax and the inspiratory film is nondiagnostic, many clinicians will obtain expiratory films (45). The diagnosis of pneumothorax can also be established with ultrasound (47) (see Chapter 6). It is important to realize that the presence of air in a hemithorax does not always represent a pneumothorax. If abdominal viscera herniate through a diaphragm, intravisceral air can be confused with pneumothorax (48). Chest CT scans are not routinely indicated in patients with primary spontaneous pneumothoraces since there is no close correlation between the presence of subpleural blebs and the recurrence of pneumothorax (49,50,51).
Approximately 10% to 20% of patients have an associated pleural effusion, which is usually small and is manifested radiographically as an air-fluid level (10,36). The pleural fluid with pneumothorax is characterized by eosinophilia, and the median percentage of eosinophils exceeds 20% after 1 day and 60% after 7 days (52). There is a significant correlation between the interleukin 5 (IL-5) levels in the pleural fluid and pleural fluid eosinophilia (52). When pneumothoraces are induced in mice, the number of eosinophils in the pleural space is reduced in tumour necrosis factor (TNF)-alpha knockout mice and in wild type mice who are given dexamethasone (53). In a small percentage of patients, the pleural effusion turns out to be a hemothorax that can be associated with life-threatening hemorrhage (54) (see Chapter 25).
Quantitation
One should estimate the amount of lung collapse when treating a patient with a pneumothorax. The volume of the lung and the hemithorax are roughly proportional to the cube of their diameters. Therefore, one can estimate the degree of collapse by measuring an average diameter of the lung and the hemithorax, cubing these diameters, and finding the ratios.
Mathematically,
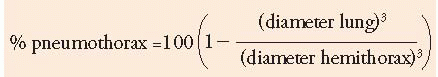
For example, in Figure 6.12, the average diameter of the hemithorax is approximately 10 cm, and the distance between the lung and chest wall is 4 cm. Therefore, the ratio of the diameters cubed 63:103 equals 22% and approximately a 80% pneumothorax is present, although it appears substantially smaller at first glance. This method of estimating the size of primary spontaneous pneumothorax has been called the Light index (55). Noppen et al. have demonstrated that there is a close correlation between the Light index and the actual amount of air in the pleural space, as quantitated by manual aspiration (55).
Collins et al. (56) have described an alternate method for estimating the percentage of collapse. With their method, the distance between the apex of the partially collapsed lung and the apex of the thoracic cavity (distance A), and the midpoints of the upper (distance B) and lower (distance C) halves of the collapsed lung and the lateral chest wall were measured in centimeters. They found that the percentage pneumothorax size could be calculated by the formula,
% pneumothorax = 4.2 + [4.7 × (A + B + C)]
When the volume calculated from a helical CT scan was compared with the volume measured with this formula, the correlation coefficient in 20 patients was 0.98 (56). Even though the correlation coefficient
was very high, improvements can be made in the preceding formula because it does not take into account the patient’s size. Obviously, a very large person will have a smaller pneumothorax in relation to the overall size of the lung than a small person with identical distances between the lung and the chest wall.
was very high, improvements can be made in the preceding formula because it does not take into account the patient’s size. Obviously, a very large person will have a smaller pneumothorax in relation to the overall size of the lung than a small person with identical distances between the lung and the chest wall.
A third method for estimating the size of a pneumothorax, the Rhea method, uses a nomogram that relates the average intrapleural distance to the pneumothorax size (57). On this nomogram, there is a 10% pneumothorax for every centimeter of intrapleural distance. A recent article found that there was a close correlation when the Collins method and the Rhea method were used to calculate pneumothorax percentage (58). However, there is no close agreement between the Collins method and the Light index (59). In general, the Rhea method or the Collins method is recommended.
The size of a pneumothorax can also be calculated from a CT scan of the thorax (60). Cai et al. (60) demonstrated that was very close agreement for the volume of a pneumothorax calculated via a computer from the chest CT scan and the volume of air aspirated from the hemithorax.
Position papers have used much simpler schemes for semiquantitating the size of pneumothoraces. In the British Thoracic Society’s (BTS) guidelines for the management of spontaneous pneumothorax, small pneumothoraces were defined as those in which the rim of air between the pleura and the chest wall at the level of the hilum was less than 2 cm and large as greater than 2 cm (61). In their consensus statement of the management of spontaneous pneumothorax, the American College of Chest Physicians defined a small pneumothorax as one in which the apex-to-cupola distance was less than 3 cm whereas a large pneumothorax was one in which this distance was greater than 3 cm (62). Collins method and Rhea method are preferred to measuring just the apex-to-cupola distance because they give a more precise estimate of the size of the pneumothorax.
Recurrence Rates
A patient who has had a primary spontaneous pneumothorax is at risk of having a recurrence. Sadikot et al. (63) followed up 153 patients with primary spontaneous pneumothorax for a mean of 54 months and found that the recurrence rate was 54.2%. In this study, the recurrence rates were less in men (46%) than in women (71%) and were less in individuals who stopped smoking (40%) than in those who continued smoking (70%). There was no significant relationship between the size of the original pneumothorax or the treatment of the original pneumothorax and the recurrence rates. Twenty-four of their patients (16%) had a pneumothorax on the contralateral side; in only one patient did the pneumothoraces occur simultaneously (63). Gobbel et al. (3) followed a group of 119 patients with spontaneous pneumothorax for a mean of 6 years. These investigators found that, of the 110 patients who did not have a thoracotomy at the time of their initial pneumothorax, 57 (52%) had an ipsilateral recurrence. Once a patient had second and third pneumothoraces without thoracotomy, the incidence of subsequent recurrence was 62% and 83%, respectively.
Older studies suggested that there is substantial risk of recurrence over many years. In the series of Gobbel et al. (3), the average interval between the first and the second pneumothorax was 2.3 years, although the average interval for recurrence in the series of Seremetis was 17 months (10). However, more recent studies have suggested that most recurrences occur within the first year (49,64,65).
Attempts have been made to predict which patients with a primary spontaneous pneumothorax are more likely to have recurrence. If one could predict which patients are more likely to have a recurrence, then those patients could be treated more aggressively to prevent a recurrent pneumothorax at the time of their first pneumothorax. The presence of blebs or bullae on chest CT scan does not predict whether the patient will develop a recurrent pneumothorax (49,51). Abolnik et al. (29) did report that taller, thinner individuals were more likely to have a recurrence. Guo et al. (66), using a multivariate analysis of 138 patients, demonstrated that recurrences were more frequent in taller patients and patients with lower weights (66).
In a recent study, Ganesalingam et al. (67) carefully studied the chest radiographs taken on the initial presentation of 100 patients for spontaneous pneumothorax for pleural thickening, blebs/bullae, pleural irregularities, and pleural adhesions. Over a mean follow-up period of 57 months, 52% of the patients had a recurrence. Patients having one, two, and three or more abnormalities were 3.0, 5.3, and 12.6 times more likely to develop a recurrence, respectively (67). They recommended that surgical treatment be offered to patients in whom two or more radiological abnormalities were identified (67).
Treatment
Therapy for the patient with primary spontaneous pneumothorax has two goals: (a) to rid the pleural
space of its air and (b) to decrease the likelihood of a recurrence.
space of its air and (b) to decrease the likelihood of a recurrence.
Several different treatments can be used for the management of a patient with a primary spontaneous pneumothorax. These include observation; supplemental oxygen; simple aspiration; tube thoracostomy with or without the instillation of a sclerosing agent; medical thoracoscopy with the insufflation of talc; video-assisted thoracoscopy with stapling of blebs, instillation of a sclerosing agent, or pleural abrasion; and open thoracotomy. In selecting the appropriate treatment for any given patient, it should be remembered that a primary spontaneous pneumothorax is mainly a nuisance and is rarely life threatening to the patient. In the following sections, discussions of the various treatments are provided. At the end of the section, recommendations for the management of primary spontaneous pneumothorax are given.
Observation
If the communication between the alveoli and the pleural space is eliminated, the air in the pleural space will be reabsorbed for the reasons discussed in Chapter 2. The rate of spontaneous absorption is slow, however. Kircher and Swartzel (68) estimated that 1.25% of the volume of the hemithorax was absorbed every 24 hours. Therefore, a pneumothorax occupying 15% of the hemithorax would take 12 days to be completely reabsorbed. In another study, Kelly et al. (69) used the Collins method to assess the rate of absorption of pneumothoraces. They reported that the mean rate of absorption was 2.2% per day, but there was much variation from patient to patient (69). It is not clear whether some of their patients were on oxygen which would increase the rate of absorption.
It is recommended that only patients with pneumothoraces occupying less than 15% of the hemithorax be considered for this type of treatment. If the patient is hospitalized, supplemental oxygen should be administered to increase the rate of pleural air absorption.
Supplemental Oxygen
The administration of supplemental oxygen accelerates the rate of pleural air absorption in experimental and clinical situations. Chernick and Avery (70) administered humidified 100% oxygen to rabbits with experimentally induced pneumothoraces and found that the oxygen increased the rate of air absorption by a factor of 6. Northfield (71) reported that the rate of absorption was increased fourfold when patients were treated with high-flow supplemental oxygen through a face mask. It is recommended that hospitalized patients with any type of pneumothorax who are not subjected to aspiration or tube thoracostomy be treated with supplemental oxygen at high concentrations.
Aspiration
The initial treatment for most patients with primary spontaneous pneumothoraces greater than 15% of the volume of the hemithorax should probably be simple aspiration (61,72,73,74). With this procedure, a 16-gauge needle with an internal polyethylene catheter is inserted into the second anterior intercostal space at the midclavicular line after local anesthesia. An alternate site is selected if the pneumothorax is loculated or if adhesions are present. After the needle is inserted, it is extracted from the cannula. Alternatively, one of the commercially available thoracentesis trays such as the Arrow-Clark Thoracentesis Kit manufactured by Arrow International or the Argyle Turkel Safety Thoracentesis Set distributed by Sherwood can be used. These kits have an outer cannula with an inner needle. If they are used, it is important to make a large-enough skin incision so that the catheter does not become crumpled during its insertion.
A three-way stopcock and a 60-mL syringe are then attached to the catheter. Air is manually withdrawn until no more can be aspirated. If no resistance has been felt after aspirating a total of 4 L, it is assumed that no expansion has occurred, and a tube thoracostomy is performed. After no more air can be aspirated, the stopcock is closed and the catheter is secured to the chest wall. After 4 hours of observation, a chest radiograph should be obtained. If adequate expansion persists, the catheter can be removed and the patient discharged. Patients should return in 24 to 72 hours for a follow-up chest radiograph.
One multicenter, prospective, randomized study compared manual aspiration versus chest tube drainage for the first episode of primary spontaneous pneumothorax. Sixty patients were randomized and immediate success was obtained in 16 of 27 patients (59.3%) in the manual aspiration group and 28 of 33 (85%) in the chest tube drainage group (72). Importantly, 13 of the 27 manual aspiration patients did not require hospitalization (72). Devanand et al. (75) performed a meta-analysis of three randomized controlled studies comparing manual aspiration and chest tube drainage and concluded that simple aspiration is advantageous in the initial management of primary spontaneous pneumothorax because of a shorter hospitalization. A recent study randomized
137 patients with their first episode of primary spontaneous pneumothorax to aspiration versus tube thoracostomy (74). The aspiration was initially successful in 40 of the 65 patients (62%). Only 17 of the 65 patients in the aspiration group were admitted to the hospital, and their mean hospital stay was 1.8 days. All patients in the tube thoracostomy group were admitted with a mean stay of 4.04 days (74).
137 patients with their first episode of primary spontaneous pneumothorax to aspiration versus tube thoracostomy (74). The aspiration was initially successful in 40 of the 65 patients (62%). Only 17 of the 65 patients in the aspiration group were admitted to the hospital, and their mean hospital stay was 1.8 days. All patients in the tube thoracostomy group were admitted with a mean stay of 4.04 days (74).
One might worry that the use of manual aspiration would be associated with a higher recurrence rate in the patients who were successfully aspirated. This does not appear to be the case. For the patients in the study of Noppen (72), the recurrence rate in patients successfully treated with aspiration was 3 of 16 (19%), whereas it was 4 of 11 (36%) in patients in whom the aspiration was unsuccessful (73). In a recent study, Ayed et al. (74) reported that the recurrence rate over 2 years was 29% in 40 patients in whom aspiration was successful compared with 25% in 72 patients who were treated with tube thoracostomy. One might hypothesize that the patients in whom aspiration is successful have smaller blebs than those in whom it fails (73). One study (76) reported that the administration of 300 mg minocycline after a successful air aspiration reduced the incidence recurrent pneumothorax from 11 of 33 (33%) to 4 of 31 (12.9%).
One complication that has been reported with anterior needle aspiration of pneumothoraces is life-threatening hemorrhage (77). In one report, three cases were described that developed life-threatening hemorrhage after the procedure (77). I know of no other similar reports, but the authors of this article suggested that it would be preferable to do the aspiration in the fifth intercostal space in the anterior axillary line (77).
If aspiration fails, the two primary alternatives are tube thoracostomy and VATS. Chen et al. (78) reported that the recurrence rates were less after VATS and the duration of hospitalization was less.
Tube Thoracostomy
With tube thoracostomy, the air in the pleural space can be rapidly evacuated. The chest tube should be positioned in the uppermost part of the pleural space, where residual air accumulates. The management of chest tubes in general is discussed in Chapter 29. Tube thoracostomy effectively evacuates the pleural air if the tube is properly inserted. In one series of 81 patients, only 3 patients (4%) had persistent air leaks after several days of chest tube drainage (10). The average duration of hospitalization in this series was only 4 days, with a range of 3 to 6 days. Although one might think that the placement of chest tubes would irritate the pleura and produce at least a partial pleurodesis, diminishing the likelihood of a recurrent pneumothorax, the incidence of recurrent pneumothorax is similar whether the initial episode is treated by bed rest alone or by tube thoracostomy (10).
When patients with spontaneous pneumothorax are managed with tube thoracostomy, several questions need to be addressed, such as what size of tube needs to be used, whether the patient can be managed as an outpatient, whether to apply suction, when to remove the tube, and when to resort to more aggressive therapy. Although one earlier study (79) concluded that the success rates were poor when patients with spontaneous pneumothorax were treated with small chest tubes (13 F), subsequent studies have reported that most pneumothoraces are effectively managed with small chest tubes (80,81,82). Minami et al. (80) treated 71 episodes of spontaneous pneumothorax using a small caliber catheter (No. 5.5 or 7.0 F) connected to a Heimlich valve. They reported that the treatment was successful in 60 patients (84.5%) and ineffective in the remaining 11 patients. Only 6 of these latter 11 patients were successfully managed when a large chest tube was placed (80). Liu et al. (82) retrospectively reviewed the results of treating 102 patients with spontaneous pneumothoraces with 8- to 10-Fr pigtail catheters or conventional chest tubes. They demonstrated that the results with both treatments were comparable (82).
When patients with pneumothorax are treated with tube thoracostomy, small tubes (7-14 F) should be tried initially because their insertion is much less traumatic than that of larger tubes (61,62,83). They are best inserted using a guidewire technique, as described in Chapter 28. If the lung does not reexpand with the small tube, then a larger tube can be inserted; however, it appears that most patients can be successfully managed with the small tube.
Patients with primary spontaneous pneumothorax can be managed with tube thoracostomy on an outpatient basis (84,85). Ponn et al. (84) inserted 12-F or 16-F short catheters in 96 patients with spontaneous pneumothorax. To prevent kinking, the tube was placed intracorporeally for most of its length, with only 1 or 2 cm plus the flared end left outside the body. A Heimlich valve was connected and secured with tape. An occlusive dressing covered the entry site, and a gauze sponge was secured over the open end of the valve with a rubber band. Patients returned every 2 to 5 days for physical examination and a chest radiograph. Using this procedure,
92 of 96 patients (96%) were treated successfully. Dernevik et al. (85) reported that they managed 31 of 35 patients (88.5%) on an outpatient basis with a device called a Tru-Close Thoracic Vent (Uresil, Sweden). This device is self-contained and consists of a chest tube, a Heimlich valve, and a thoracic vent (85). The cost reduction associated with outpatient management is obvious; most patients with primary spontaneous pneumothorax who are subjected to tube thoracostomy should be treated as outpatients.
92 of 96 patients (96%) were treated successfully. Dernevik et al. (85) reported that they managed 31 of 35 patients (88.5%) on an outpatient basis with a device called a Tru-Close Thoracic Vent (Uresil, Sweden). This device is self-contained and consists of a chest tube, a Heimlich valve, and a thoracic vent (85). The cost reduction associated with outpatient management is obvious; most patients with primary spontaneous pneumothorax who are subjected to tube thoracostomy should be treated as outpatients.
It is recommended that no suction be applied to chest tubes inserted for spontaneous pneumothorax (61). The chest tubes can either be connected to a Heimlich valve or an underwater seal. Two studies (79,86) have concluded that the rate at which the lung reexpands is similar whether suction is applied or not. Because the risk of reexpansion pulmonary edema is greater when suction is applied to the chest tube (87), and because the suction appears to offer no benefit, suction is not recommended. In one series (88), reexpansion pulmonary edema occurred in 16 of 84 patients (19%) with spontaneous pneumothorax who had -20 cm H2O suction applied to the chest tube. Although this is a much higher incidence than in most other studies, it does emphasize the recommendation to not use suction. If the lung does not expand after 24 hours of water-seal drainage or Heimlich valve drainage, suction should be applied to the chest tube. A Heimlich valve comes with some of the commercially available kits (see Chapter 28). It is important to hook up the Heimlich valve in the right direction, or a tension pneumothorax can result (89).
The chest tube should remain in place for 24 hours after the lung reexpands and the air leak ceases. If the chest tubes are removed too soon after the lung reexpands and the air leak ceases, there is a high likelihood of an early recurrence. Sharma et al. (86) reported a recollapse rate of 25% in 20 patients in whom the chest tubes were removed within 6 hours of lung expansion, but a recollapse rate of 0% in 20 patients in whom the chest tubes were removed 48 hours after lung expansion. There is controversy as to whether the chest tube should be clamped if the lung has reexpanded and if there is no air leak. The thought behind this procedure is that if there is a small air leak that is not obvious, then a small pneumothorax will develop if the tube is clamped and then chest tube drainage can be reinitiated. There are no studies to my knowledge evaluating how many pneumothoraces will be detected with this approach. Chest tubes should certainly not be clamped if there is an air leak because clamping in this situation could lead to a tension pneumothorax.
Not all primary spontaneous pneumothoraces are treated successfully with a small chest tube. If the patient is initially treated with a small caliber chest tube and the lung does not expand within 48 hours, a larger chest tube should be placed. If the lung has not expanded or a bronchopleural fistula persists after 3 or 4 days, consideration should be given to more invasive therapy such as thoracoscopy or thoracotomy or to performing an autologous blood patch (see later sections in this chapter). The insertion of additional chest tubes is not recommended (90).
Tube Thoracostomy with Instillation of a Sclerosing Agent
Approximately 50% of patients with an initial primary spontaneous pneumothorax have a recurrence whether they are treated with observation, aspiration, or tube thoracostomy. Efforts have been made to diminish the recurrence rates by injecting various agents into the pleural space in an attempt to create an intense inflammatory reaction that would obliterate the pleural space. Many different materials including quinacrine (91), talc slurry (92), olive oil (93), and tetracycline (65,94) have been instilled through the chest tube at the time of the initial pneumothorax in an effort to create a pleurodesis and prevent a recurrence.
The two agents that appear to be the best sclerosing agents are talc slurry and the tetracycline derivatives. Most commonly, when talc is used as an agent to affect a pleurodesis, it is insufflated at the time of thoracoscopy or thoracotomy (see the discussion in the following text). There have been two reports (92,95), however, with a total of 32 patients in whom 5 to 10 g of talc suspended in 250 mL of saline was administered intrapleurally. The recurrence rate in these 32 patients was less than 10%.
The primary drawback to using talc is that a small percentage of patients develop the acute respiratory distress syndrome (ARDS) from its instillation intrapleurally (96). The mortality rate is not inconsequential. In one recent study in which talc slurry was used to treat 240 patients with malignant pleural effusion, the incidence of respiratory failure was 4%, and 5 (2%) of the patients died (97). Although most deaths following talc slurry have occurred in patients who were being treated for malignant pleural effusions rather than pneumothorax, the fact that ARDS and death do occur after administering talc slurry intrapleurally should make one hesitant to use it for a benign condition in young healthy individuals (96). Talc insufflation for prevention of pneumothorax
recurrence has also been associated with the development of chronic debilitating pain requiring thoracotomy (98). A more in-depth discussion of the side effects of talc can be found in Chapter 10.
recurrence has also been associated with the development of chronic debilitating pain requiring thoracotomy (98). A more in-depth discussion of the side effects of talc can be found in Chapter 10.
An alternative agent for pleurodesis in patients with pneumothorax is a tetracycline derivative. In the Veterans Administration (VA) cooperative study on pneumothorax, 229 patients who were being treated with tube thoracostomy for spontaneous pneumothorax were randomized to receive 1,500 mg of tetracycline or nothing through their chest tube. During the 5-year study period, the 25% recurrence rate in the tetracycline group was significantly less than the 41% recurrence rate in the control group (65). In a second study, Alfageme et al. (99) reported that the recurrence rate was 9% in 66 patients treated with tetracycline intrapleurally, whereas the recurrence rate was 36% for the 79 patients treated with observation or chest tubes only (99). In a more recent study of 138 patients that was not randomized, Guo et al. used proportional hazards analysis and demonstrated that the administration of a sclerosing agent (tetracycline 45 patients, gentamicin 23 patients) was associated with a significantly lower recurrence rate (66).
In summary, the evidence presented earlier strongly suggests that the intrapleural injection of a sclerosing agent in patients with spontaneous pneumothorax significantly reduces the subsequent recurrence rates. It appears that at least in rabbits, the effectiveness of the pleural sclerosant is decreased if the animal is receiving corticosteroids (100,101). The administration of the nonsteroidal anti-inflammatory agent diclofenac (2 mg/kg body weight) also decreased the effectiveness of pleurodesis from mechanical abrasion in pigs (102). Therefore, one should attempt to minimize corticosteroids and anti-inflammatory agents in patients in whom pleurodesis is attempted. Which patients with spontaneous pneumothorax should receive the intrapleural injection of an agent in an attempt to produce a pleurodesis and decrease recurrence rates? It is recommended that all patients with primary or secondary spontaneous pneumothorax who are treated with tube thoracostomy receive an agent unless they are subjected to thoracoscopy or thoracotomy.
What agent should be used? Currently, the recommended agent for an attempted pleurodesis through a chest tube is a tetracycline derivative. If it were not for the reported cases of ARDS after the administration of talc slurry to patients with malignant pleural effusions, talc slurry would be the recommended agent. Parenteral tetracycline is no longer available in most countries due to increasingly stringent manufacturing requirements for parenteral antibiotics. Tetracycline derivatives appear comparable in effectiveness to tetracycline. In the rabbit model, minocycline (103) or doxycycline (104) is as effective as tetracycline in producing a pleurodesis at approximately one fourth the dose of tetracycline. Intrapleural doxycycline is also an effective treatment for malignant pleural effusion (see Chapter 10). Accordingly, 500 mg of doxycycline intrapleurally is recommended for patients with spontaneous pneumothorax who are treated with chest tubes. An alternative agent is minocycline (300 mg intrapleurally) (78). Bleomycin should not be used because it is ineffective in producing a pleurodesis in rabbits with a normal pleura (105) and expensive.
The intrapleural injection of tetracycline derivatives is an intensely painful experience for many patients. In the VA cooperative study (65), more than 50% of the patients reported severe pain at the time of the tetracycline injection, and 70% of the individuals stated that the pain was greater at the time of the tetracycline injection than at either the onset of pneumothorax or at the time the chest tube was placed. The intrapleural administration of 100 mg of lidocaine (Xylocaine) was not effective in ameliorating the intense chest pain. However, some have recommended that when a tetracycline derivative is administered intrapleurally for pneumothorax, the injection be preceded by 4 mg/kg of Xylocaine up to a maximal dose of 250 mg (106). The patient should also be premedicated with an agent such as a short-acting benzodiazepine (e.g., midazolam).
It is recommended that the tetracycline derivative be injected as soon as the lung has reexpanded. The patient should be positioned so that the tetracycline comes into contact with the apical pleura. In experimental animals, the presence of a small pneumothorax at the time of the injection does not decrease the efficacy of the pleurodesis (107). A persistent air leak is not a contraindication to tetracycline injection. There is, however, no evidence that the intrapleural injection of tetracycline leads to an earlier closure of the bronchopleural fistula (65,108).
Autologous Blood Patch for Persistent Air Leak
In the past decade, there have been approximately 50 papers reporting on the use of the autologous blood patch for the treatment of a persistent air leak in patients with spontaneous pneumothorax (109,110). With this technique, 50 to 100 mL of blood is drawn from a vein and then promptly injected, without anti-coagulation, through the chest tube into the pleural
space (109). The chest tube is elevated about 60 cm to prevent the blood from being drained. The chest tube should not be clamped because with the airleak this could lead to a tension pneumothorax. Chambers et al. (109) reviewed the literature on utilizing the blood patch technique for airleaks in conjunction with pneumothorax and reported that the airleak ceased in 91.7% of 107 patients. It is possible that the blood-patch technique might also decrease the incidence of recurrence. In the study of Cagirici (111), there were no recurrences in any of the 32 patients treated with the blood-patch technique during a follow-up of 12 to 48 months. When the blood-patch technique has been used in patients undergoing needle aspiration of the lung, it was ineffective in preventing pneumothorax in two studies (112,113), but it was effective in preventing larger pneumothoraces in the most recent study (114).
space (109). The chest tube is elevated about 60 cm to prevent the blood from being drained. The chest tube should not be clamped because with the airleak this could lead to a tension pneumothorax. Chambers et al. (109) reviewed the literature on utilizing the blood patch technique for airleaks in conjunction with pneumothorax and reported that the airleak ceased in 91.7% of 107 patients. It is possible that the blood-patch technique might also decrease the incidence of recurrence. In the study of Cagirici (111), there were no recurrences in any of the 32 patients treated with the blood-patch technique during a follow-up of 12 to 48 months. When the blood-patch technique has been used in patients undergoing needle aspiration of the lung, it was ineffective in preventing pneumothorax in two studies (112,113), but it was effective in preventing larger pneumothoraces in the most recent study (114).
Other agents have been injected intrapleurally in an attempt to stop the airleak. Cobanoglu et al. evaluated 50 patients with persistent airleaks from spontaneous pneumothorax and reported that the airleak stopped in 30 of 40 (75%) with the blood patch, in 16 of 19 (84%) with talc, and in 6 of 11 with tetracycline. They concluded that the blood patch was the technique of choice because the airleak ceased more quickly and that it had less effect on the pulmonary function (115).
Intrapleural Fibrin Glue for Persistent Air Leak
There has been one article (116) that suggested that the intrapleural administration of a large amount of diluted fibrin glue might be effective in patients with persistent air leaks. Kinoshita et al. (116) diluted both the compounds used with regular fibrin glue to 60 mL and then injected the 120 mL total into 40 high-risk patients with persistent bronchopleural fistula. They reported that the bronchopleural fistula closed after one injection in 35 patients, after two injections in 4 patients, and after three injections in 1 patient. The air leaks ceased within 12 hours of injection. During the follow-up period of 2.5 to 6.5 years, the pneumothorax recurred in five patients (12.5%), but an additional single treatment with fibrin glue resulted in resolution of the pneumothorax with no further recurrences (116). If these results can be duplicated by others, the intrapleural administration of fibrin glue represents a significant advance in the treatment of high-risk patients with pneumothorax. However, since this article was published in 2003, I have been unable to find additional articles using intrapleural fibrin glue to treat airleaks with spontaneous pneumothorax.
Medical Thoracoscopy
Medical thoracoscopy is performed with the patient under local anesthesia, usually combined with conscious sedation (see Chapter 30). In contrast, video-assisted thoracoscopic surgery (VATS) is performed almost exclusively under general anesthesia with double-lumen endotracheal intubation, which allows single-lung ventilation and the collapse of the lung on the operated side (117). However, there is one report (118) in which VATS was performed with the patient awake using spinal anesthesia. There are strong advocates for using medical thoracoscopy for the treatment of primary spontaneous pneumothorax (117,119,120,121). Tschopp et al. (119) treated 89 patients for persistent or recurrent spontaneous pneumothorax from 1986 to 1994 with talc insufflation at the time of medical thoracoscopy. They reported that the initial medical thoracoscopy was successful in 80 patients (90%) and that the subsequent recurrence rate was 7.5% (6 of 80 patients). In a second study, 108 patients were randomized to receive medical thoracoscopy with talc insufflation or tube thoracostomy (120). In this study, the recurrence rate over 5 years in the patients who received talc was 5% and the authors concluded that medical thoracoscopy was more cost-effective than drainage alone (120). There have been reports that talc insufflation was associated with the development of ARDS and death. However, a recent report (122) on 418 patients who received talc insufflation with graded talc reported no instances of ARDS.
The primary difference between medical thoracoscopy with talc insufflation and VATS is that with medical thoracoscopy no attempt is made to treat the blebs. The advocates of medical thoracoscopy do not believe that the blebs are important in the pathogenesis of the pneumothorax (121). However, there are no randomized controlled studies comparing these various techniques. Nevertheless, the general consensus is that recurrence rates are slightly higher with medical thoracoscopy (˜5%) than with VATS (˜3%) (123). One advantage that medical thoracoscopy has over VATS is that it is much less expensive.
Video-Assisted Thoracoscopic Surgery
VATS is effective in the treatment of spontaneous pneumothorax and the prevention of recurrent pneumothorax. With VATS, there are two primary objectives: (a) to treat the bullous disease responsible for the pneumothorax and (b) to create a pleurodesis. Currently, the most common means by which bullae
are treated is with an endoscopic stapling device. The primary disadvantage of the endostapler is its expense. The Endo-GIA model costs approximately US$500, and additional cartridges (of which an average of two per procedure are used) cost US$500 each (124). Previously, the bullae were treated with electrocoagulation, which was associated with a higher recurrence rate (125). An alternative method of dealing with the apical bullae is to ligate the bullae with a Roeder loop (126). However, Inderbitzi et al. (126), who have reported one of the largest series using VATS for the treatment of pneumothorax, have reported a relatively high recurrence rate after use of the loop and recommend that it be abandoned in favor of wedge resection with the endostapler. One series concluded that endostapling of the lung apex was associated with a decreased recurrence rate even if no blebs are visible (127). The recurrence rate during a mean follow-up of 38.7 months was 7% in the 57 patients who did not receive the stapling, whereas there were no recurrences in the 69 patients who received the stapling (127).
are treated is with an endoscopic stapling device. The primary disadvantage of the endostapler is its expense. The Endo-GIA model costs approximately US$500, and additional cartridges (of which an average of two per procedure are used) cost US$500 each (124). Previously, the bullae were treated with electrocoagulation, which was associated with a higher recurrence rate (125). An alternative method of dealing with the apical bullae is to ligate the bullae with a Roeder loop (126). However, Inderbitzi et al. (126), who have reported one of the largest series using VATS for the treatment of pneumothorax, have reported a relatively high recurrence rate after use of the loop and recommend that it be abandoned in favor of wedge resection with the endostapler. One series concluded that endostapling of the lung apex was associated with a decreased recurrence rate even if no blebs are visible (127). The recurrence rate during a mean follow-up of 38.7 months was 7% in the 57 patients who did not receive the stapling, whereas there were no recurrences in the 69 patients who received the stapling (127).
Once the lesion in the lung is treated, some attempt should be made to create a pleurodesis. Recurrence rates are higher when only the blebs are treated (128,129). The primary alternatives are mechanical abrasion of the pleura, partial parietal pleurectomy, talc insufflation, and argon beam coagulation (130). Of these four, mechanical abrasion of the pleura is the simplest. In one study (131) of 569 patients treated with VATS for spontaneous pneumothorax, the recurrence rates over a 5-year follow-up period were 3.6% for abrasion, 1.1% for poudrage, and 2.5% for pleurectomy, which did not differ significantly. Since there is no other evidence that partial pleurectomy, talc poudrage, or argon beam coagulation is associated with less recurrences than mechanical abrasion, it is the method of choice. In another approach, Marcheix et al. (132) treated 603 patients with stapling of the blebs and spraying 1% silver nitrate on the parietal pleural and reported that the long-term recurrence rate was only 1.1%.
There have been several series, each with more than 100 patients, in which patients with spontaneous pneumothorax were treated with VATS. In general, VATS with stapling of bullae is very effective at managing spontaneous pneumothorax, with an overall recurrence rate of approximately 3% (133,134,135,136,137). Yim and Liu (133) treated 483 patients with VATS using mechanical pleurodesis plus some other procedures such as endostapling or endoloop for management of the bullae. They reported that their median postoperative hospital stay was only 3 days and the recurrence rate was 1.74%, with a mean follow-up of 20 months (133). Cardillo et al. (134) used VATS to treat 432 patients with primary spontaneous pneumothorax between 1992 and 1998. They used subtotal pleurectomy to induce a pleurodesis in some patients and talc insufflation in others. The conversion rate to open procedures was 2.3%, most often due to extensive pleural adhesions. The mean time to chest tube removal was 5.4 days, and the mean hospital stay was 6.1 days. The recurrence rate was 4.4%, with a mean follow-up of 38 months (134). In a more recent study, Margolis et al. (138) performed VATS with stapling of blebs and pleural abrasion in 156 young (median age 19) patients with their first spontaneous pneumothorax and reported no recurrences with a mean follow-up of 62 months. The mean total hospital stay was 2.4 days (138).
Cardillo et al. (139) reported the largest series ever reported from a single institution. They treated 861 patients with VATS and talc poudrage. If the patients had no visible blebs, they were treated with only talc poudrage. If blebs or bullae were visible, they were stapled (139). After a mean follow-up of 52.5 months, the recurrence rate was 2.4% in the group without blebs and 1.7% in the group with blebs (139). Since I would expect the group with no blebs to have less recurrences, this study suggests to me that treatment of the blebs is important.
One paper randomly assigned 202 patients to resection of blebs plus pleural abrasion or resection of blebs plus pleural abrasion plus the instillation of 300 to 400 mg minocycline at the end of the procedure (140). The group of patients that received minocycline had more pain postoperatively, but there was a significant decrease in the recurrence rate for pneumothorax (1.9% vs. 8.1%) (140). However, it should be noted that the recurrence rates in the patients treated with resection of blebs plus pleural abrasion is much higher than in most series. Accordingly, minocycline in addition to pleural abrasion is not recommended currently.
Patients with hemopneumothoraces in which there is significant pleural hemorrhage are probably best managed with VATS. Hwong et al. (141) performed VATS on 25 patients with spontaneous hemopneumothorax and reported that the bleeding was controlled in all the patients. VATS also appears to be effective in patients who have had a recurrence after talc insufflation with medical thoracoscopy (142). Doddoli et al. (142) successfully managed 27 of 39 such patients (69%) with VATS. VATS is also an effective management strategy
for recurrent pneumothorax after a previous VATS procedure (143,144,145).
for recurrent pneumothorax after a previous VATS procedure (143,144,145).
Open Thoracotomy
The indications for open thoracotomy are the same as those for thoracoscopy. If VATS is available, thoracotomy is recommended only after VATS has failed. The reason for this recommendation is that the hospitalization is shorter and the postoperative pain is less severe after thoracoscopy (124,146,147). However, in a meta-analysis of 29 studies (148) the recurrence rate after open thoracotomy (1.1%) was significantly lower than that after VATS (5.4%), but many of the studies were done when thoracoscopy was first being used for pneumothorax and recurrence rates fell as the surgeons became more experienced. It should be mentioned, however, that some surgeons still prefer axillary mini-thoracotomy to VATS for the treatment of spontaneous pneumothorax (148,149). The reason for this preference is that time is saved because double-lumen intubation is not required, the operating time is short, there is a good cosmetic result, and it is less expensive (149).
At thoracotomy, the apical pleural blebs are oversewn and the pleura is scarified. This procedure is effective in controlling the pneumothorax and diminishing the rate of recurrence. In one large series in which 362 patients underwent parietal pleurectomy, only two documented ipsilateral recurrences were reported, with an average follow-up of 4.5 years in 310 patients (150). The low rates of morbidity and mortality of the procedure are attested to in the same article (150). Only 1 operative death was reported in the 362 operative procedures, and the average postoperative period of hospitalization was only 6 days. Various methods proposed for scarification of the pleura range from visceral and parietal pleurectomy to mere abrasion of the pleura with dry sponges. All of these procedures appear to be effective (8), but because pleural abrasion with dry gauze is less traumatic than pleurectomy and does not affect a potential later thoracotomy, it is the procedure of choice.
Air Travel
If a patient has a spontaneous pneumothorax, when should they be allowed to travel by air? Commercial airlines have adopted a 6-week “no fly” rule between pneumothorax occurrence and air travel (151). This rule seems very arbitrary and there are no data to support it. Currie et al. (152) reported two patients with loculated pneumothoraces who flew without incident. I believe that the 6-week “no fly” rule is very conservative, and I will allow my patients to fly 7 days after resolution of the pneumothorax.
Recommendations
My recommendations for the management of a patient with a primary spontaneous pneumothorax are as follows. If the pneumothorax is small (<15% of the hemithorax) and the patient is asymptomatic, observation is recommended. If the patient is at a site where oxygen is available, high-flow supplemental oxygen should be administered. If the pneumothorax is >15% of the hemithorax, an attempt should be made to aspirate the pneumothorax. If this is successful and there is no recurrence over several hours, the patient can be sent home. If the aspiration is unsuccessful, then the patient should be admitted to the hospital. If possible, thoracoscopy should be performed as soon as possible. Thoracoscopy is preferred to tube thoracostomy because it is associated with less recurrences, a shorter mean hospital stay, and a shorter duration of chest tube drainage (153). In general, if both medical thoracoscopy and VATS are available, VATS is preferred because with it the apical blebs can be treated. If thoracoscopy is not available, tube thoracostomy should be performed with the injection of a sclerosing agent. The sclerosing agent of choice is doxycycline 500 mg. Talc is not recommended because its administration intrapleurally can lead to ARDS that can be fatal. If a patient has a recurrent pneumothorax, VATS or medical thoracoscopy should be performed. VATS or medical thoracoscopy should also be performed if the patient has an occupation, for example, airplane piloting, or an avocation, for example, diving, where the occurrence of a pneumothorax might be life threatening. VATS or medical thoracoscopy is not indicated if the aspiration is successful because most such patients will never have a recurrence.
SECONDARY SPONTANEOUS PNEUMOTHORAX
Secondary spontaneous pneumothoraces are more serious than primary spontaneous pneumothoraces because they decrease the pulmonary function of a patient with already compromised pulmonary function. The secondary spontaneous pneumothoraces that occur in patients with the acquired immunodeficiency syndrome (AIDS), cystic fibrosis, tuberculosis, lymphangioleiomyomatosis (LAM), and Langerhans cell histiocytosis are discussed in separate sections.
Incidence
The incidence of secondary spontaneous pneumothorax is similar to that of primary spontaneous pneumothorax. In the study from Olmsted County, Minnesota, the incidence was 6.3 and 2.0/100,000/year for men and women, respectively (1). If these figures are extrapolated to the entire population of the United States, approximately 10,000 new cases of secondary spontaneous pneumothorax will be seen each year. In a more recent study from the United Kingdom, the incidence of spontaneous pneumothorax for males and females above 55 was 32.4 and 10.9/100,000/year, respectively (2). Interestingly, the incidence in men kept increasing as the age increased (2).
Etiologic Factors
Most secondary spontaneous pneumothoraces are due to COPD, although almost every lung disease has been reported to be associated with secondary spontaneous pneumothorax. In one series of 505 patients from Israel with secondary spontaneous pneumothorax, the etiologies were as follows: COPD, 348; tumor, 93; sarcoidosis, 26; tuberculosis, 9; other pulmonary infections, 16; and miscellaneous, 13 (154).
There appears to be a tendency for patients with more severe COPD to develop spontaneous pneumothorax. In the VA cooperative study, which included 171 patients with secondary spontaneous pneumothorax, 51 of the patients (30%) had a forced expiratory volume1 (FEV1) <1,000 mL and 56 of the patients (33%) had an FEV1/forced vital capacity (FVC) <0.40 (65).
Clinical Manifestations
In general, the clinical symptoms associated with secondary spontaneous pneumothorax are more severe than those associated with primary spontaneous pneumothorax. Most patients with secondary spontaneous pneumothorax have dyspnea (155,156), which frequently seems out of proportion to the size of the pneumothorax (157). In one series of 57 patients with COPD, all complained of shortness of breath, whereas 42 (74%) had chest pain on the side of the pneumothorax (155). In addition, five patients were cyanotic and four patients were hypotensive.
The occurrence of a pneumothorax in a patient with underlying lung disease is a serious event. Because the pulmonary reserve of these patients is already diminished, the partial or total loss of the function of a lung can be life threatening. In one series of 18 patients in whom arterial blood gases were obtained at the time of admission, the mean Pao2 was 48 mm Hg and the mean Paco2 was 58 mm Hg (155). In the VA cooperative study, the Pao2 was below 55 mm Hg in 20 of 118 (17%) and was below 45 mm Hg in 5 of 118 (4%). The Paco2 exceeded 50 mm Hg in 19 of 118 (16%) and exceeded 60 mm Hg in 5 of 118 (4%) (65).
A substantial mortality rate is associated with secondary spontaneous pneumothorax. When three older series totaling 120 patients are combined, the mortality rate was 16% (155,157,158). Causes of death included sudden death before chest tubes could be inserted in three patients, respiratory failure within the first 24 hours of treatment in three patients, late respiratory failure in three patients, and massive gastrointestinal bleeding in three patients. However, in the VA cooperative study, none of the 185 patients with secondary spontaneous pneumothorax died from a recurrent ipsilateral pneumothorax. However, the overall mortality rate in the 5-year follow-up period was 43% (65). The high mortality rate probably reflects the severity of the underlying disease. The leading causes of death were COPD, lung cancer, pneumonia, and heart disease (65).
The physical examination of patients with secondary spontaneous pneumothorax is less helpful than it is in primary spontaneous pneumothorax. These patients already have hyperexpanded lungs, decreased tactile fremitus, hyperresonant percussion notes, and distant breath sounds over both lung fields. Accordingly, when a pneumothorax develops, side-to-side differences in the physical examination may not be apparent. The possibility of a pneumothorax should be considered in any patient with COPD who has increasing shortness of breath, particularly if chest pain is also present.
Diagnosis
As with primary spontaneous pneumothorax, the diagnosis of secondary spontaneous pneumothorax is established by the chest radiograph. In patients with COPD, the radiographic appearance of the pneumothorax is altered by the loss of elastic recoil of the lung and the presence of air trapping. Normal areas of the lung collapse more completely than diseased areas with large bullae or severe emphysema in the absence of adhesions. In addition, the deflation of the diseased lung is limited by its decreased elastic recoil. Although ultrasound can be used to establish the
diagnosis of pneumothorax in patients with primary spontaneous pneumothorax and traumatic pneumothorax, it is less reliable in patients with COPD (159). If pleural gliding is present, one can be confident that there is no pneumothorax. However, pleural gliding is sometimes absent in patients with COPD who do not have a pneumothorax (159).
diagnosis of pneumothorax in patients with primary spontaneous pneumothorax and traumatic pneumothorax, it is less reliable in patients with COPD (159). If pleural gliding is present, one can be confident that there is no pneumothorax. However, pleural gliding is sometimes absent in patients with COPD who do not have a pneumothorax (159).
The diagnosis of pneumothorax is established by the demonstration of a visceral pleural line. It is sometimes difficult to see this line because the lung is hyperlucent and little difference exists in radiodensity between the pneumothorax and the emphysematous lung. Frequently, the presence of the pneumothorax is overlooked on the initial chest radiograph. One must distinguish a spontaneous pneumothorax from a large, thin-walled, air-containing bulla. The pleural line with a pneumothorax is usually oriented in convex fashion toward the lateral chest wall, whereas the apparent pleural line with a large bulla is usually concave toward the lateral chest wall. If there is any doubt as to whether the patient has a pneumothorax or a giant bulla, CT scan should be obtained because the two conditions are easily differentiated with this procedure (45,160). It is important to make the distinction between a large bulla and a pneumothorax because only the pneumothorax should be treated with tube thoracostomy.
In patients with cystic lung disease, the presence of cysts and pleural adhesions sometimes makes it difficult to determine whether a pneumothorax is present on the routine chest radiographs. If patients with cystic lung disease present with increased shortness of breath, the possibility of a pneumothorax should be considered, particularly if the hemithoraces are asymmetric in size. In such cases, the CT scan will delineate whether a pneumothorax is present and will also assist in selecting the appropriate site for chest tube placement (161).
Occasionally, secondary spontaneous pneumothoraces result from primary carcinoma of the lung with bronchial obstruction. One must recognize the radiologic signs of bronchial obstruction in these patients because the insertion of chest tubes is contraindicated. When a patient has a totally collapsed lung, one should search for air bronchograms in the lung. Air bronchograms are absent when there is an obstructing endobronchial lesion, but otherwise they are present (162). If no air bronchograms are present, a bronchoscopic examination should be performed before a chest tube is inserted.
Recurrence Rates
The recurrence rates for secondary spontaneous pneumothorax appear to be somewhat higher than those for primary spontaneous pneumothorax (64,65,163). Videm et al. (163) followed a total of 303 patients for a median period of 5.5 years and reported that 24 of the 54 patients (44%) with COPD had a recurrence. In patients without COPD, 96 of 249 (39%) had a recurrence (163). In the VA cooperative study, 92 patients with secondary spontaneous pneumothorax were treated with chest tubes without pleural sclerosis and the recurrence rate was 47% with a median follow-up of 3 years (65). In this study, the recurrence rate with primary spontaneous pneumothorax was 32% (65). Guo et al. in a multivariate analysis of the factors related to recurrent pneumothorax found that patients with secondary spontaneous pneumothoraces were significantly (p < 0.007) more likely than patients with primary spontaneous pneumothorax to have a recurrence (66).
Treatment
The goals of treatment of the patient with secondary spontaneous pneumothorax, as with primary spontaneous pneumothorax, are to rid the pleural space of air and to decrease the likelihood of a recurrence. Achievement of these goals, particularly the second, is more important in the patient with secondary spontaneous pneumothorax, however. A primary spontaneous pneumothorax or its recurrence is mostly just a nuisance. In contrast, the occurrence of a pneumothorax in a patient with lung disease may be life threatening, even though the mortality rate from recurrent secondary spontaneous pneumothorax is low (65).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


