Pneumonia in the Severely Immunocompromised Patient
Introduction
Lung infection in patients with suppressed or impaired immune function is a common clinical problem. The majority of these patients have relatively impaired function secondary to age, common conditions such as diabetes mellitus, or treatment for chronic disease with corticosteroid therapy. These patients have an increased risk of lung infection but typically this is with common bacterial organisms that may also be seen in the wider population and can be investigated and managed in a similar fashion. Infection with the more uncommon organisms that are usually associated with the immunocompromised patient are relatively rare and confined to those with severely impaired immune function, commonly patients with acquired immune deficiency syndrome (AIDS), those on immunosuppressive therapy, or patients undergoing chemotherapy for malignancy. In this chapter an algorithm is presented for the investigation of severely immunocompromised patients with suspected lung infection and the clinical, radiological, microbiological, and pathological features of infection with cytomegalovirus (CMV), Pneumocystis carinii, Aspergillus spp., and Candida spp. are discussed, with recommendations on possible treatment regimens.
Aetiology
Pneumonia in patients with severe neutropenia (e.g. bone marrow transplantation, chemotherapy for malignancies) is often due to bacteria, such as Pseudomonas aeruginosa, Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, other ‘coliforms’ and fungi such as Aspergillus fumigatus and Candida albicans. Patients with predominantly T-lymphocyte deficiencies, such as in AIDS, or with therapeutically-induced immunodeficiency states, such as after solid organ transplantation, are more often associated with infections due to CMV or Pneumocystis carinii.
There is considerable overlap between these two groups, and infections due to rarer organisms (see Chapter 7) often occur in immunocompromised patients.
Presentation
Immunocompromised patients may present in a wide range of paediatric and adult clinical settings. Classification of immunodeficiency states is as follows:
Primary:
– Antibody defects/deficiencies.
– Cell-mediated defects.
– Combined defects.
Acquired/secondary:
– Immunosuppressive therapy.
– Chemotherapy.
– Underlying malignancy.
– HIV infection.
– Steroid therapy.
– Extremes of age.
– Diabetes mellitus.
While the more severe primary defects invariably present in childhood, some of the less severe forms, such as IgG subclass deficiencies, may not present until adulthood and must be considered in patients with persistent or recurrent chest infections. The commonest causes of secondary immunodeficiency states are extremes of age, diabetes, steroid therapy, immunosuppressant therapy, and intercurrent illness, particularly malignancy. Infection with the more unusual organisms such as P. carinii, CMV, and invasive fungal infections are, however, usually seen in the more severely immunosuppressed patient such as those receiving chemotherapy or immunosuppressive agents, or with HIV infection.
Investigations
Figure 3.1 presents a flow chart of the standard investigations used in the care of the immunocompromised patient.
The following specific infections in the immunocompromised patient will be discussed:
CMV.
P. carinii.
Aspergillus spp. (usually A. fumigatus).
Candida spp. (usually C. albicans).
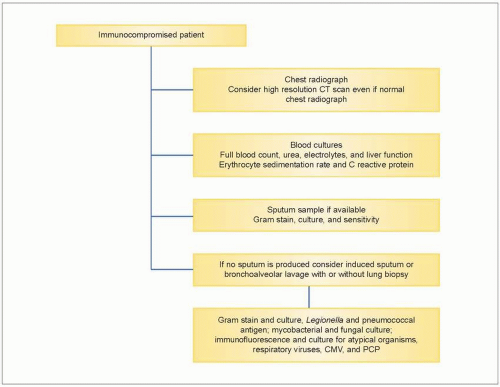 3.1 Flow chart of standard investigations used in the immunocompromised patient suspected of having pneumonia. |
CMV pneumonitis
CMV pneumonitis presents in up to 40% of recipients of solid organ transplants. Mortality rates are high, at up to 50% even with treatment. Clinical signs are not specific for CMV pneumonitis, with patients usually presenting with unproductive cough, breathlessness, pyrexia, tachypnoea, and hypoxaemia.
Investigations
Blood tests
A full blood count may show neutropenia and reduced platelet count. Liver function tests may be deranged. Inflammatory markers are usually raised, with an elevated erythrocyte sedimentation rate and C-reactive protein. Arterial blood gases often show hypoxaemia with a type 1 respiratory failure.
Radiology
Chest radiographic changes are not specific for CMV pneumonitis, and may be subtle or even absent. Changes are usually bilateral, with diffuse or focal opacification or diffuse interstitial infiltrates. Similarly, changes seen on high resolution CT scans of the chest are not specific but commonly show diffuse, multiple, small nodules, patchy areas of dense consolidation, and ‘ground glass’ attenuation.
Illustrative case
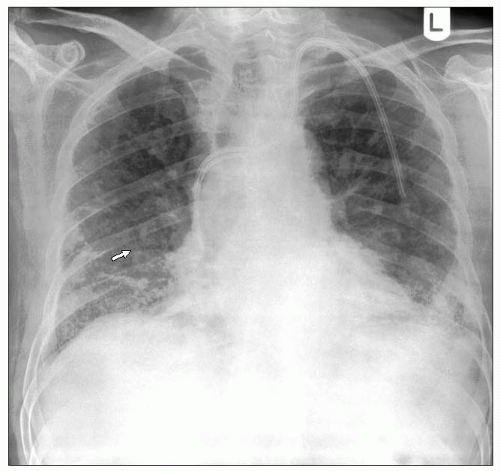
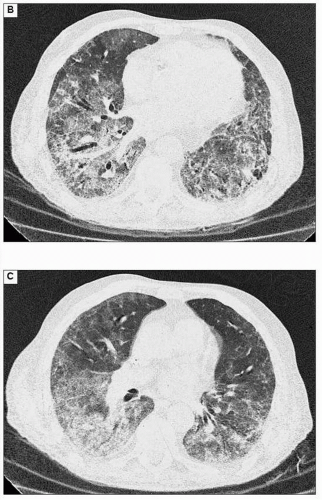
Figures 3.2A is a chest radiograph from a 74-year-old male with renal failure due to microscopic polyarteritis, on immunosuppressant therapy. He developed a fever and increased breathlessness. The chest radiograph shows widespread interstitial shadowing thought initially to be due to fluid overload but he did not respond to treatment. Note also the Portacath in situ for dialysis. The CT scans (3.2B, C) show a mixture of ground glass changes, areas with consolidation, and mild interlobular septal thickening. There is a predominant peripheral distribution and more marked disease in the mid and lower zones. This was confirmed as CMV pneumonitis.
Pathology
Figure 3.3 is a photomicrograph of a lung showing an acute CMV pneumonia. The lung shows foci of necrotizing inflammation with destruction of the alveolar architecture. Severe necrotizing pneumonia in profoundly immunosuppressed patients may arise as a result of bacterial, fungal, or viral infection. Identification of the aetiological agent may be possible histologically, but in some cases this will not be obvious and the results of microbiological investigation will be required to make a diagnosis. A higher power photomicrograph of the same specimen (3.4) shows the typical ‘owl’s eye’ nuclear inclusions seen with CMV infection. When the specimen is stained immunohistochemically with a monoclonal antibody to CMV, infected cells stain brown (3.5). This shows intense nuclear and weaker cytoplasmic staining of the cell, reflecting the distribution of the viral antigen.
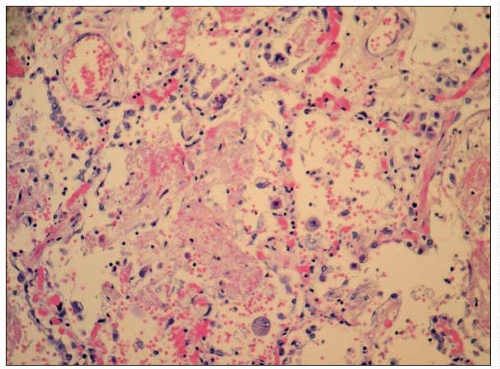 3.3 Photomicrograph of a lung showing foci of necrotizing inflammation with destruction of the alveolar architecture, due to an acute CMV pneumonia. |
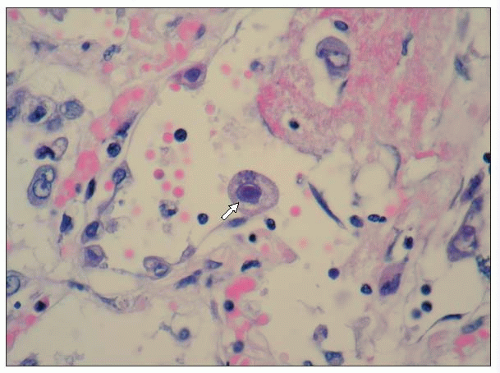 3.4 High power micrograph of the specimen in 3.3, showing the typical ‘owl’s eye’ nuclear inclusions seen with CMV infection (arrow). |
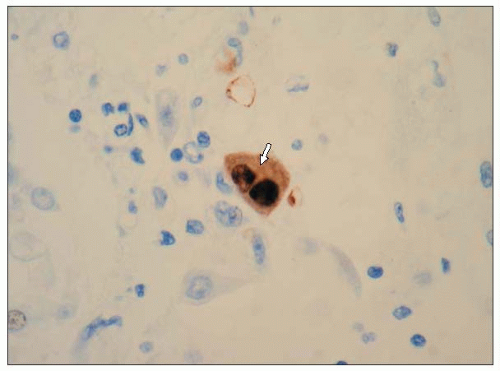 3.5 Immunohistochemical staining of the specimen in 3.3 with a monoclonal antibody to CMV, showing intense nuclear and weaker cytoplasmic brown staining of the cell, reflecting the distribution of the viral antigen (arrow). |
CMV infection is very common, but severe clinical manifestations usually occur only in immunocompromised individuals. Interstitial pneumonitis is the commonest organ specific manifestation in patients with AIDS or after organ or bone marrow transplantation. Other co-existing or competing pathogens are frequently found (e.g. HIV pneumonitis, PCP).
Spontaneous sputum is not an appropriate sample for the diagnosis of CMV pneumonia. Definitive diagnosis requires laboratory examination of induced sputum, bronchoalveolar lavage, lung biopsy, or buffy coat from anticoagulated blood samples. Laboratory examination must include tissue culture to achieve maximal sensitivity and specificity, although a number of newer methods allow more rapid diagnosis. These latter methods include immunofluorescence using monoclonal antibodies to detect early antigens in tissue cultures or tissue samples, and nucleic acid probes on respiratory and tissue samples and on peripheral blood buffy coat to detect CMV-specific DNA or RNA sequences. Serology may also be helpful: rising titres indicate recent infection, and raised IgM titres suggest active infection.
Treatment
The decision to treat can be difficult; isolation of CMV from either induced sputum or bronchoalveolar lavage fluid is not sufficient alone to determine treatment. If, however, in addition there is an associated pneumonitis from the chest radiograph or the CT scans of the chest then treatment would be recommended. The optimum investigations would include the combination of bronchoalveolar lavage fluid with transbronchial lung biopsies and PCR from venous blood. By combining these test results with radiology, the decision to treat is facilitated. In active infection patients who would normally be expected to show radiological changes, CMV would be detected by PCR from blood and/or bronchoalveolar lavage and there would be typical histological features of CMV pneumonitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree