Chapter 69 Pleural Effusion, Empyema, and Pneumothorax
Pleural Effusion
Epidemiology and Pathophysiology
Epidemiology
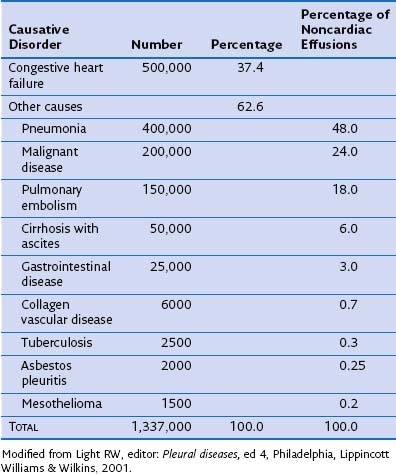
More than 60 causes of pleural effusions have been documented. The relative incidence of different types of effusion varies according to patient demographics and geographic areas. Heart failure is responsible for approximately one third of all pleural effusions (Table 69-1), with pleural infection and malignancy accounting for most exudative effusions.
Imaging
Thoracic Ultrasound Imaging
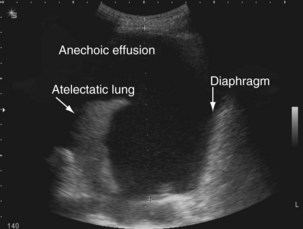
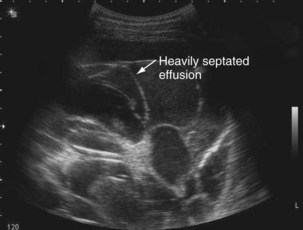
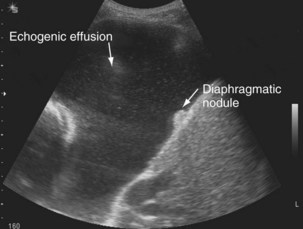
The availability of bedside thoracic ultrasound examination by clinicians has had a significant impact on pleural disease management in recent years. The 2010 British Thoracic Society Pleural Disease Guidelines strongly recommended the use of thoracic ultrasound imaging before procedures for pleural fluid. It is particularly useful for the detection (sensitivity approximately 100%), quantification (by depth), and characterization of pleural fluid (Figures 69-1 to 69-3; Table 69-2), as well as for guiding intervention. Ultrasonography is invaluable in the differentiation between pleural fluid and collapsed or consolidated lung, thereby avoiding unnecessary pleural procedures and associated complications.
Table 69-2 Pleural Fluid Sonographic Appearances
| Sonographic Appearance | Significance |
|---|---|
| Anechoic (black fluid) (see Figure 69-1) | Transudative or exudative effusion |
| Septated (multiple lines within fluid) (see Figure 69-2) | Exudative effusion; may suggest possible difficulties inserting chest tube; effusion may drain poorly, although not necessarily |
| Echogenic (echoes, often swirling, within fluid) (see Figure 69-3) | Exudative effusion; heavily echogenic fluid suggestive of blood or pus |
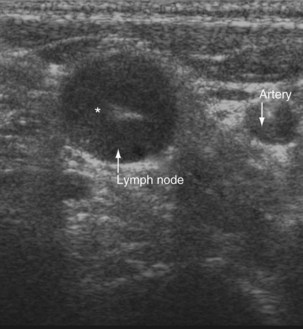
Ultrasound imaging has a high sensitivity (approximately 80%) for detecting pleural malignancy, which can manifest as thickening or nodularity on the visceral, parietal, and diaphragmatic pleural surfaces (see Figure 69-3). Detection of pleural nodularity mandates further investigation (e.g., with chest computed tomography [CT] and pleural biopsy), even if there are no further suspicious features. Ultrasonography also can identify abnormalities beyond the pleural cavity that may provide vital clues to the cause of the effusion, including peripheral lung tumors or abscesses, parenchymal consolidation and atelectasis, diaphragmatic paralysis or elevation, pericardial effusion, and rib and liver metastases and enables evaluation of supraclavicular and cervical lymphadenopathy (Figure 69-4).
Computed Tomography and Magnetic Resonance Imaging
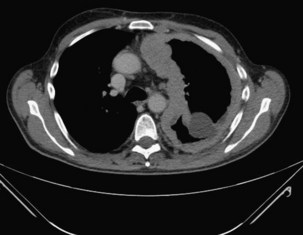
CT with pleural phase contrast enhancement highlights pleural abnormalities and aids discrimination of benign from malignant disease (see Chapter 7). Specific “pleural” CT protocols should be adopted for optimal pleural enhancement and abnormality detection; recent data suggest that images should be acquired 60 seconds after injection of 150 mL of an intravenous contrast agent at 2.5 mL/second. The presence of contraction of the hemithorax, mediastinal pleural involvement, and circumferential pleural thickening (especially greater than 1 cm and with nodularity) all are suggestive of pleural malignancy (Figure 69-5) but cannot adequately differentiate mesothelioma from metastatic pleural cancers. Magnetic resonance imaging (MRI) can help delineate malignant chest wall involvement and is valuable in selected cases, particularly when (probably benign) pleural abnormalities are to be followed clinically by serial imaging in younger patients.
Positron Emission Tomography
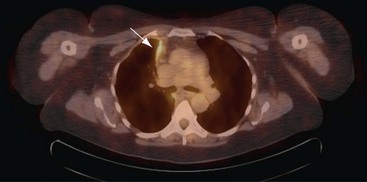
Positron emission tomography (PET)-CT scanning (see Chapter 8) is beginning to emerge as a useful tool in pleural disease management. PET-CT cannot adequately differentiate between benign and malignant effusions, because of the tracer 18F-fluorodeoxyglucose (FDG). FDG-enhanced PET imaging is confounded by avid pleural uptake of FDG in the presence of pleural inflammation (including that due to previous talc pleurodesis and pleural infection). However, FDG-PET may have a role in guiding pleural biopsy in patients with diffuse pleural abnormality to increase sensitivity (Figure 69-6). FDG-PET also may identify nonpleural sites that allow tissue sampling to confirm malignancy (e.g., lymphadenopathy or liver metastases). Recent data suggest a role for FDG-PET in monitoring disease response to therapy in malignant mesothelioma, as well as a potential prognostic role.
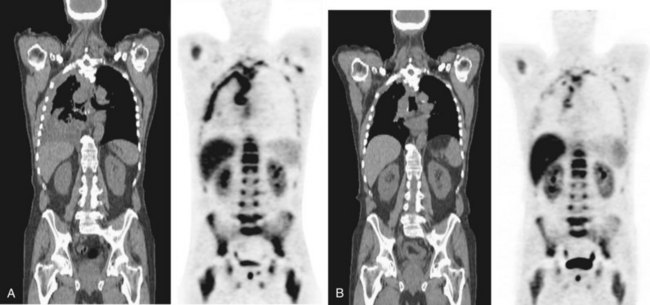
PET scanning using various novel molecular tracers is in early-phase trials for evaluation of pleural malignancies. For instance, PET scanning using labeled thymidine, essential for deoxyribonucleic acid (DNA) synthesis, can identify sites of cell proliferation activity and is not confounded by inflammation (Figure 69-7). New tracers targeting specific cell biology processes (e.g., annexin, a marker of apoptosis) are likely to provide valuable insight to disease pathobiology.
Diagnostic Approach
Investigation of a pleural effusion should be performed using a systematic approach (Figure 69-8), aiming to minimize the number of pleural procedures required to make a diagnosis and thereafter allow definitive treatment.
Thoracentesis, preferably imaging-guided, should be the initial investigation in pleural effusions of uncertain origin. If small (less than 1 cm in depth) effusions require sampling, this procedure should be undertaken using real-time radiologic guidance. Thoracentesis is generally safe and complications are uncommon but include vasovagal syncope (0.6%), pneumothorax, infection, and bleeding. Removal of large amounts of fluid may precipitate reexpansion pulmonary edema, often heralded by cough, chest discomfort (at which point the procedure must be terminated), or acute dyspnea. Pleural manometry has been advocated but is not widely available. If initial pleural fluid analysis is inconclusive, additional investigations are often required, including further imaging, repeat thoracentesis, and thoracoscopic or percutaneous pleural biopsy (see Chapters 13 and 74).
Pleural Fluid Analysis
Separation of Exudates and Transudates
Exudative pleural effusions most commonly are defined by Light’s criteria (Box 69-1), using the fluid-to-serum ratio of protein and lactate dehydrogenase, which has an accuracy of 96%. Numerous other markers and criteria have been tested (including measurement of pleural fluid cholesterol values), but none has proved superior. Distinguishing exudates from transudates may narrow the scope of the differential diagnosis and streamline further investigations, although such categorization has limitations: It does not provide the diagnosis and fails to identify concurrent transudative and exudative causes of fluid formation. Research in recent years has focused on disease-specific markers that may provide a definitive diagnosis.
Box 69-1
Light’s Criteria
A pleural fluid is an exudate if any of the following criteria are met:
1. Pleural fluid–to–serum protein ratio greater than 0.5
2. Pleural fluid–to–serum lactate dehydrogenase (LDH) ratio greater than 0.6
3. Pleural fluid LDH more than two-thirds the upper limit of normal serum LDH
Differential Leukocyte Count
The cellular portion of physiologic pleural fluid consists predominantly of macrophages and monocytes. In disease states, the differential cell count of the pleural fluid may be helpful in determining the cause (Box 69-2). Acute pleural inflammation or injury generates chemotaxins, such as interleukin 8, and attracts neutrophils to the pleural space. A neutrophil-predominant effusion is commonly seen with acute bacterial pneumonia or pulmonary infarction. A lymphocyte-rich fluid is more common in disease of insidious onset such as tuberculosis (TB) or malignancy. Tuberculous effusions occasionally (less than 10%) may be neutrophilic. An increased eosinophil count (more than 10% of total leukocytes) is often nonspecific. Most commonly, eosinophil effusions develop secondary to presence of intrapleural air or blood (including pneumothorax or previous interventions) but can also be associated with a range of other diseases, such as Churg-Strauss syndrome or drug-induced pleuritis.
pH and Glucose
Pleural fluid pH (or glucose) measurement can aid disease management. Low glucose levels are associated with a similar spectrum of diseases that give rise to low pH effusions (e.g., infection and connective tissue diseases) (Box 69-3) and are equally informative except in patients with hyperglycemia.
Box 69-3
Conditions Commonly Associated With Low-pH and Low-Glucose Pleural Effusions*
Pleural Biopsy
Histologic examination (with or without culture) of pleural tissue can aid in the diagnosis of specific pleural diseases. Tissue can be collected percutaneously (by “blind” or imaging-guided biopsy) or under direct vision (by thoracoscopy or thoracotomy), each with its relative merits (see Chapters 13 and 74).
Thoracoscopy
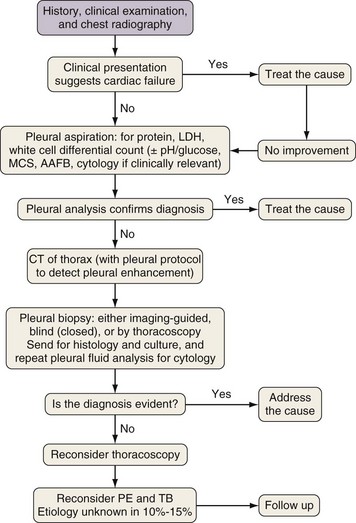
Thoracoscopy is indicated in patients with an undiagnosed exudative pleural effusion after a nondiagnostic thoracentesis. Thoracoscopy can be performed by physicians or surgeons either using local anesthesia and sedation (pleuroscopy) or with the patient under general anesthesia using single-lung ventilation (video-assisted thoracoscopic surgery, [VATS]). Pleuroscopy allows direct visualization of almost the entire pleural surface (Figure 69-9), drainage of fluid, parietal pleural biopsy, and pleurodesis by talc poudrage in the same procedure. Diagnostic sensitivity approaches 95% in malignant pleural diseases and almost 100% in tuberculous pleurisy. Complications are few, and mortality rates low (less than 0.5%). VATS has a similarly high diagnostic sensitivity but also allows other invasive interventions during surgery, including lung biopsy. For details of the technique, refer to Chapter 74.
Treatment
Symptom Control
Placement of Indwelling Pleural Catheters
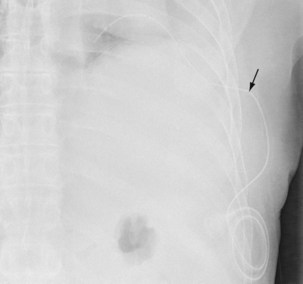
The insertion of a long-term tunneled indwelling pleural catheter (IPC) allows outpatient drainage of refractory malignant pleural effusions. The ambulatory catheters can be inserted as a “day case,” avoiding the need for hospital admission. Its presence induces a complete or partial pleurodesis in up to 70% of patients, and the patient (or the caregiver) can drain the effusion as guided by symptoms, averting hospitalization. Implantation of an IPC also is indicated in patients with a symptomatic effusion and underlying trapped lung, as described later. The catheters usually are well tolerated in clinical practice, but reported complications include development of pleural septations, pleural or soft tissue infection, local tumor invasion at the insertion site, and catheter displacement (Figure 69-10). A randomized trial is under way to evaluate the use of IPCs as first-line therapy in malignant effusions.