Spontaneous
Primary
Secondary
– Chronic obstructive pulmonary disease
– Bronchiolitis
– Pneumocystis infection
– Cystic fibrosis
– Asthma
– Necrotizing pneumonia
– Tuberculosis infection
– Congenital cysts
– Pulmonary fibrosis
Catamenial
Traumatic
Blunt injury
Penetrating injury
Iatrogenic
Barotrauma—mechanical ventilation
Thoracentesis
Percutaneous transthoracic lung biopsy
Central venous catheterization
Thoracotomy/thoracostomy
Others
Bronchopleural fistula
Esophageal perforation
Spontaneous Pneumothorax: no underlying trauma or iatrogenic cause for pneumothorax
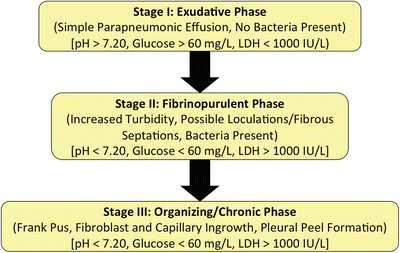
Primary Spontaneous Pneumothorax: no known underlying lung disease (Fig. 4.1)
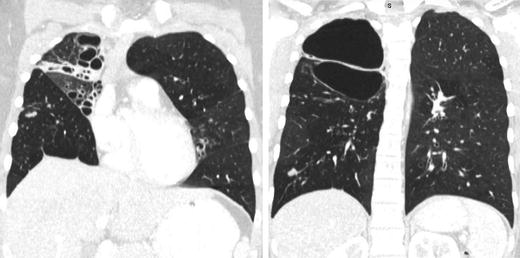
Fig. 4.1.
Chest CT of a 27-year-old patient with severe bullous disease. This patient suffered from recurrent primary spontaneous pneumothoraces.
Incidence 7.4/100,000 (men) and 1.2/100,000 (women) annually in the USA [3].
Caused by rupture of small bleb (bullae) usually in apices of upper or lower lobes, allowing air to leak into the pleural cavity.
80 % of patients will demonstrate emphysema-like changes on CT scan [4].
Occur most commonly in thin tall young male patients. Smoking and atmospheric pressures changes are also risk factors [5].
Secondary Spontaneous Pneumothorax: known underlying lung disease.
Incidence 6.3/100,000 (men) and 2.0/100,000 (women) annually in the USA [3].
Underlying disease causing rupture and air leak
Clinical Presentation
Pleuritic chest pain and dyspnea (most common)
Physical examination may be normal if the pneumothorax is <25 %.
Decreased breath sounds, hyperresonance on the affected side (not always present)
Subcutaneous emphysema
Tension pneumothorax also presents with tracheal deviation to the contralateral side, severe respiratory distress and haemodynamic instability.
Rarely: pneumomediastinum or pneumopericardium (Hamman’s sign)
Diagnosis is established by an upright chest X-ray (Fig. 4.2). If clinical signs of tension physiology are evident, X-ray confirmation should be omitted and immediate decompression should ensue.
Fig. 4.2.
Patient presenting with a traumatic pneumothorax after blunt trauma to the chest. Black arrows denote the outline of the collapsed lung.
Expiratory view accentuates the separation of the parietal and visceral pleura.
Pneumothorax in supine patients accumulates into the dependent regions of the anterior and subdiaphragmatic pleura and may be detected as a deep sulcus sign.
Although CT scan is the gold-standard for diagnosis, it is not necessary for the majority of patients with first-episode spontaneous pneumothorax since it does not change the management.
Management
The aetiology largely dictates both immediate and definitive management.
Observation:
Reserved for asymptomatic patients with a small pneumothorax who are unlikely to have an ongoing air leak. Follow-up radiography should be obtained within 24–48 h to document improvement.
Supplemental oxygen can help decrease the alveolar pressure of nitrogen in the body, thus creating a gradient to reabsorb the air from the pleura (mostly composed of nitrogen) into the alveoli and tissues.
Conservative management re-expands the lung at an average rate of 2.2 %/day with a 79 % success rate [8].
Failure for the pneumothorax to resolve may lead to fibrothorax (fibrous entrapment of the lung).
Aspiration:
A small cannula can be used to aspirate pleural air, with a success rate of 75 and 40 % for a primary and secondary spontaneous pneumothorax, respectively. This technique is rarely used.
Can be attached to a Heimlich one-way valve or a three-way stop-cock with a large syringe [9].
Percutaneous Catheters:
Small-calibre tube thoracostomy can be performed percutaneously via the Seldinger technique and attached to either a Heimlich one-way valve or suction.
Use is limited to a spontaneous pneumothorax with limited respiratory symptoms.
Tube Thoracostomy:
Large-bore chest tubes are the standard-of-care for treatment of traumatic pneumothoraces, unstable patients, persistent or large air leaks and associated effusions or haemothoraces.
Clamping of the tube is controversial. If performed, a follow-up chest radiograph should be done in several hours to assess for re-accumulation.
Outpatient management is acceptable with the use of a Heimlich one-way valve [10].
Complications of chest tube insertion include: injury to the lung, intercostal vessels, or great vessels, misplacement in the fissures or outside the pleural cavity, infection, and re-expansion pulmonary edema caused with rapid re-expansion leading to increase in capillary permeability.
The appropriate initial management option should be tailored to the expected size of the air leak.
Conservative options include observation, needle aspiration, and small-bore tube thoracostomy connected to an underwater seal. For persistent or larger air leaks, large-bore chest tubes with underwater seal connected to wall suction can be used, with surgery and/or pleurodesis as last resorts [11].
Recurrence after spontaneous pneuothorax is 30 % after the first episode, with the majority occurring within 2 years [12]. Independent risk factors for recurrence include: pulmonary fibrosis, age >60 years, increased height/weight ratio [13]. Recurrence rate increases after each episode.
Surgery:
Guidelines recommend waiting at least 3–5 days for resolution of a spontaneous pneumothorax before considering definitive surgical management [14].
A bullectomy can be performed preferably via a VATS approach with a success rate >95 % [15]. Other options including open thoracotomy and axillary approaches.
Indications for bullectomy [14]:
After the second spontaneous primary pneumothorax
After the first spontaneous primary pneumothorax in patients with high-risk professions, or patients exposed to significant changes in atmospheric pressures
After the first spontaneous secondary pneumothorax
Pleural Effusions
An abnormal collection of fluid in the pleural space
Can be transudative (low protein) or exudative (high protein).
Benign pleural effusions are twice as common as malignant pleural effusions [18] (Table 4.2).
Causes
Incidence (cases/year)
Congestive heart failure
500,000
Parapneumonic effusion/empyema
300,000
Pulmonary embolus
150,000
Viral pleuritis
100,000
Post-coronary artery bypass graft
60,000
Hepatic hydrothorax
50,000
Collagen vascular disease
6,000
Tuberculosis pleuritis
2,500
Pathophysiology [19]: See Chap. 4: Pleural Disorders (Anatomy and Physiology)
Increased pulmonary capillary pressure (CHF, renal failure)
Increased pulmonary capillary permeability (pneumonia)
Decreased intrapleural pressure (atelectasis)
Decreased plasma oncotic pressure (hypoalbuminemia)
Increased pleural permeability (infection, inflammation)
Obstruction of pleural lymphatic drainage (malignancy)
Fluid from other sites or cavities (peritoneum, retroperitoneum)
Rupture of thoracic vessels (haemothorax, chylothorax)
Drugs [18]
Work-Up (Table 4.3)
Table 4.3.
Differential diagnosis of pleural effusions.
Transudative effusions | |
Cardiovascular | Congestive heart failure |
Pulmonary embolus | |
Infradiaphragmatic | Cirrhosis |
Peritoneal dialysis | |
Other | Nephrotic syndrome |
Hypoalbuminemia (malnutrition, liver failure) | |
Exudative effusions | |
Infections (most common) | Bacterial (sepsis, pneumonia) |
Tuberculosis | |
Viral (respiratory, hepatic, cardiac) | |
Fungal | |
Parasitic | |
Neoplasm | Primary lung cancer |
Metastatic disease (lung, breast, colon and ovarian cancers most common) | |
Mesothelioma | |
Infradiaphragmatic | Pancreatitis |
Peritonitis | |
Bilothorax (biliopleural fistula, bile duct obstruction) | |
Inflammatory bowel disease | |
Intra-abdominal abscess | |
Endoscopic esophageal sclerotherapy | |
Meigs syndrome (pleural effusion and ascites with pelvic tumours) | |
Autoimmune | |
Drugs | |
Post-operative | |
Other | Amyloidosis |
Haemothorax | |
Chylothorax | |
Esophageal rupture | |
Benign asbestos-related effusion | |
Serum Laboratory: blood count and differential (high white count suggests infection, bleeding, malignancy), serum electrolytes, urea, creatinine, liver function tests and liver enzymes, albumin, lactate dehydrogenase, lipase, cardiac enzymes, electrocardiogram
Imaging:
Chest X-ray (presence of >250 mL pleural fluid [20])
Complicated pleural infections are suggested by abnormal pleural indentation that does not correspond to the effects of gravity on pleural fluid.
Ultrasound: can identify small effusions and loculations, and can guide thoracocentesis, drain placement and pleural biopsy.
CT scan: best imaging study to characterise size, location, presence of loculations and underlying cause of pleural effusions (Fig. 4.3). It can also guide chest drain placement for complicated effusions/empyema.
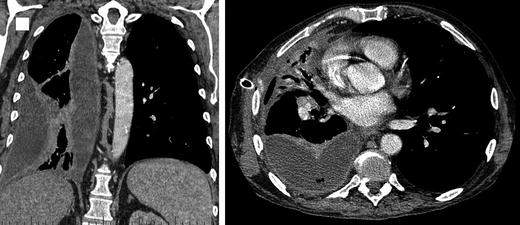
Fig. 4.3.
Patient presenting with a parapneumonic effusion that progressed to advanced stage of empyema.
Signs of pleural infection include pleural thickening, pleural space air (gas-forming organisms), and the split pleura sign in empyema (pleural fluid encased by distinct thickened visceral and parietal pleura) [21].
Pleural Fluid Sampling: unless the cause of the effusion is known (i.e. CHF) the pleural fluid should be sampled.
Pleural Fluid Characteristics [22]:
Straw colour (normal, transudate)
Turbid/purulent (empyema)
Blood (trauma, malignancy, parapneumonic)
Enteric content (esophageal rupture)
Bile (bilothorax)
Pleural Fluid Analysis: the following tests should be utilised to characterise the effusion [18, 19]:
pH (<7.2 useful for identifying complicated infected effusions)
LDH (should be measured in pleural fluid and serum)
Protein (pleural fluid and serum)
Bacterial culture (some fluid should be additionally placed in blood culture bottles to improve diagnostic accuracy [23, 24]) and Gram Stain
Cytology (to detect malignancy)
CBC (to identify WBCs from infection, or RBCs from blood)
Acid-fast bacilli (AFB) PCR (if lymphocytic effusion is found or TB is a concern especially in endemic regions)
Light’s Criteria: used to distinguish exudative from transudative pleural effusions. If 1 or more are positive, effusion is exudative [25].
Pleural Fluid/Serum Protein ratio >0.5
Pleural Fluid/Serum LHD ratio >0.6
Pleural Fluid LDH >2/3 the upper limit of normal serum LDH
Pleural Biopsy: can assist the diagnosis of tuberculosis, malignancy, and amyloidosis when diagnosis is uncertain [26].
Pleural Infections (Empyema)
Symptoms
Similar to pneumonia: pleuritic chest pain, fever, cough, dyspnea, malaise [20]
Constitutional symptoms (malaise, fatigue, weight loss, anorexia) in malignant pleural effusions
Causes
Direct contamination of pleural space (trauma, surgery)
Hematologic spread (bacteremia/sepsis)
Direct extension from lung parenchyma (parapneumonic)
Rupture of intrapulmonary abscess or infected cavity
Bronchopleural fistula
Extension from mediastinum (esophageal perforation)
Classification
Microbiology [33]:
Community Acquired (85 %):
Aerobes (73 %): Streptococci (72 %; S. milleri/anginosus (46 %), S. pneumonia (40 %)), Staphylococci (14 %; MSSA (77 %), MRSA (20 %)), gram-negative (12 %), others (2 %)
Anaerobes (22 %)
Other (5 %)
Nosocomial (15 %):
Aerobes (88 %): Staphylococci (40 %; MRSA (71 %), MSSA (29 %)), gram-negative (26 %), Streptococci (21 %), Enterococci (13 %)
Anaerobes (8 %)
Other (4 %)
Management
Drainage:
Historically large bore chest tubes (30–36 Fr) were advocated for drainage of pleural infections due to fear of tube blockage by thick viscous drainage [20].
A recent case series using 10–16 Fr tubes of pleural infections reported a 72 % success rate, comparable to large bore (>30 Fr) chest tubes [34].
A retrospective analysis of 405 patients treated with chest tube drainage of various sizes (<10 Fr, 10–14 Fr, 15–20 Fr, >20 Fr) revealed no difference in mortality or requirement for decortication [35].
No differences in chest radiograph change following drainage, length of stay or pulmonary function after 3 months were noted between tube size groups.
Irrigation of pleural cavity can be done with sterile saline following drain insertion, however evidence supporting this practice is lacking [20].
Antibiotics:
Patients often present with signs of sepsis.
Broad-spectrum antibiotic therapy covering gram-positive, gram-negative (including Pseudomonas) and anaerobic bacteria is indicated.
Focus antibiotics once cultures obtained.
Antibiotics in pleural fluid typically reach approximately 75 % of serum levels [20].
Duration of antibiotics: minimal evidence suggesting optimal duration, however therapy is generally continued for 2–4 weeks following resolution of signs and symptoms in order to achieve clinical resolution, depending on microbiology, response to therapy, extent of disease, adequacy of drainage and patient factors (e.g. immune status).
Fibrinolytic Therapy (Streptokinase/tPA +/− DNAse):
Used as treatment of loculated parapneumonic effusions and empyema.
Aimed to reduce incidence of surgical intervention.
Meta-analysis of 7 RCTs comparing fibrinolytic therapy to placebo shows reduction in surgical intervention but not in mortality or length of stay [28].
Double-blind RCT MIST2 trial evaluating t-PA and DNAse for patients with pleural infection [36].
Randomised to 1 of 4 treatments for 3 days: t-PA and DNAse, t-PA and placebo, placebo and DNAse, double placebo.
Primary outcome: change in pleural opacity (percentage of hemithorax occupied by effusion on chest X-ray [Mean (+/− SD) change in pleural opacity]).
t-PA + DNAse versus placebo:
Change in pleural opacity: −29.5 ± 23.3 % vs. −17.2 ± 19.6 %, p=0.005
Surgical referral at 3 months: OR 0.17; 95 % CI, 0.03 to 0.87; P = 0.03.
Hospital stay (mean difference): −6.7 days; 95 % CI, −12.0 to −1.9; P = 0.006.
Non-significant for t-PA or DNAse alone versus placebo.
Decortication:
Performed by open posterolateral thoracotomy or by VATS
Indications:
Stages II and III after failure of chest tube drainage, intrapleural fibrinolysis and antibiotic therapy.
Late chronic empyema with entrapped lung.
Provides surgical drainage and allows lung to re-expand eliminating potential space that harbours bacteria.
Complete decortication allows for lung re-expansion to tamponade air leak and bleeding [20, 27, 32].
VATS Decortication (VATSD) versus Open Thoracotomy Decortication (OD):

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree