The potential genetic basis of aortic stenosis in older people is poorly understood. A total of 265 patients with aortic stenosis involving tricuspid aortic valves and 961 controls were genotyped for ≤660 candidate single nucleotide polymorphisms (SNPs). After dividing the patients and controls into training and validation sets, we tested the correlation of the SNPs with the age-adjusted aortic valve area, determined by echocardiography or cardiac catheterization. A bootstrapped global p value of ≤0.005 was considered evidence of a possible significant correlation. The cases were aged 73 ± 7 years, and 72.7% were men. The median aortic valve area was 1.0 cm 2 (interquartile range 0.7 to 1.5). The controls were aged 69 ± 6 years, and 69.8% were men. The minor allele frequency was 21% ± 15% (37% <0.20). Three SNPs met the criteria for significant correlation (rs2276288 [MYO7A], p = 0.001; rs5194 [AGTR1], p = 0.004; rs207 307 [ELN], p = 0.005). Another 2 SNPs reached borderline significance (p ≤0.008). In conclusion, we report 3 SNPs to be associated with aortic stenosis involving tricuspid aortic valves in older subjects. Given the concerns regarding the problem of multiple statistical testing, validation studies are required to further assess these correlations.
Aortic stenosis (AS) involving tricuspid aortic valves occurs in up to 6% of patients >75 years old. Previously considered a “degenerative” problem, AS is now understood to be an active cellular process associated with proliferative and inflammatory changes, lipid accumulation, and osteogenic changes at the tricuspid aortic valve. Limited studies suggest familial clustering and an association with the vitamin D receptor, interleukin-10, transforming growth factor-β (TGF-β) and apolipoprotein (apo)B and apoE4 alleles. Although clinical trials of statins to slow progression of AS failed in patients with advanced disease, a better understanding of the risk factors and earlier intervention might allow the development of therapies to retard disease progression.
Methods
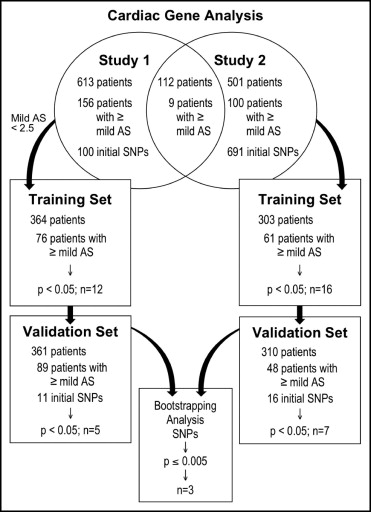
From 2001 to 2004 in an institutional review board-sanctioned registry for broad cardiovascular application, 10,000 consecutive consenting subjects undergoing cardiac catheterization for clinical indications were characterized extensively for phenotype and had DNA samples banked for future genotyping. Subgroups of patients were then genotyped under several different protocols. The present analysis represents a combination of data from 2 partially overlapping protocols that initially focused on identifying the genetic correlates of coronary artery disease. Study 1 had 1,189 and study 2 had 1,554 total patients. All of these patients with echocardiographic or catheterization-based evaluation of their aortic valve for possible AS were included in the present study. The echocardiographic and catheterization findings were updated by a review of the electronic medical record as of January 1, 2011. Patients with bicuspid or quadricuspid valves, findings most consistent with rheumatic valvular disease, or <60 years old were excluded.
Genomic DNA was isolated from the whole blood drawn into an ethylenediaminetetraacetic acid tube using an automated DNA extraction system with the Puregene DNA purification kit (Gentra Systems, Minneapolis, Minnesota) and stored at −70°C until use. In study 1, genotyping was performed by melting-curve analysis of oligonucleotide fluorescent probes designed to span over the polymorphic sites. The primers and probes designed using the probe design software supplied with the LightTyper Instrument (Roche Applied Science, Indianapolis, Indiana) were procured commercially (TIB Molbiol, Freehold, New Jersey). DNA from the Coriell Institute for Medical Research (Camden, New Jersey; available from: http://www.coriell.org ) was used to optimize and validate each candidate single nucleotide polymorphism (SNP) assay before genotyping the patient DNA samples. Genotyping was performed using a semiautomated high-throughput process and a liquid handling system (Tecan Genesis 150, Tecan US, Durham, North Carolina) to pipette the DNA, PCR Mastermix (LightCycler FastStart DNA Master Hybridization Probes, Roche Applied Science) containing SNP-specific primers and probes. The polymerase chain reaction was performed in the twin-block GeneAmp PCR System 9700 (Applied Biosystems, Carlsbad, California) followed by melting-curve analysis using the LightTyper to decipher the genotypes. In study 2, genotyping was performed by allele-specific kinetic polymerase chain reaction using 0.3 ng of DNA. Each allele was amplified separately in the presence of SYBR Green using 1 of 2 allele-specific primers and a common reverse primer on an PRISM 7900HT Sequence Detection System (Applied Biosystems) at the Celera high-throughput genotyping facility (Alameda, California). Some SNPs were assessed by separately repeating the genotyping using an allele-specific oligonucleotide ligation assay similar to a previously reported method, and hybridization to oligonucleotides coupled to Luminex 100TM xMAP microspheres (Luminex, Austin, Texas).
In both studies, candidate SNPs were chosen for their likely association with the coronary artery disease extent and propensity toward plaque rupture. The risk factors for calcific AS have been noted to be similar to those for vascular atherosclerosis. A total of 29 SNPs from 8 genes previously reported to be associated with calcific AS or closely related genes were also studied; however, we did not study the SNPs in interleukin [IL]-10 (rs1800872) or apoB (rs6725189) that achieved the greatest level of correlation with AS in the study by Gaudreault et al.
The most recent echocardiographic or catheterization findings pertinent to AS were characterized systematically. If a prosthetic valve was in place, the previous records were reviewed to determine the findings present before surgery. The mean transvalvular gradient and calculated aortic valve areas, peak transvalvular gradient, and other echocardiographic findings were recorded. When no or a minimal aortic gradient was present and a valve area was not provided, the areas were imputed as follows: mean gradient <5 mm Hg, area considered 3.0 cm 2 ; mean gradient 5 to 8 mm Hg, area considered 2.5 cm 2 ; and mean gradient 9 to 12 mm Hg, area considered 2.0 cm 2 .
Continuous data are presented as the mean ± SD or median and interquartile range, as appropriate, and compared using the Student or Kolmogorov-Smirnov test, respectively. Categorical data are presented as the frequency and compared using chi-square analysis. Training and validation sets were determined by a random number generator, with 50% of patients intended for each group. In the training cohort, the individual allele (SNP) data were correlated with the severity of aortic valve stenosis (aortic valve area) with and without adjustment for patient age and creatinine. Candidate alleles with corrected p <0.05 were then reanalyzed in a similar fashion in the validation cohort. SNPs with p <0.05 in the validation cohort were then subjected to bootstrapping analyses applied to the entire cohort (75% sample, 1,000 repetitions) to calculate a final estimated p value. A threshold p value of 0.005 was set, considering the total number of SNPs tested, the reduced power to detect associations for SNPs with low minor allele frequency, and the limited number of patients.
Results
A schematic of the study design is depicted in Figure 1 . The baseline patient characteristics are listed in Table 1 . As is typical for most cardiac catheterization populations, most of the 1,226 patients were middle-age men with coronary artery disease. Also, 21.6% had some degree of AS. The characteristics of the patients with and without AS are listed in Table 2 . The patients were older than the control subjects and somewhat more often men or had diabetes. The minor allele frequency was 21% ± 15%. The relation between significant clinical variables and the severity of AS is listed in Table 3 .
| Characteristic | Study 1 | Study 2 | ||
|---|---|---|---|---|
| Training (n = 360) | Validation (n = 365) | Training (n = 306) | Training (n = 307) | |
| Age (years) | 72 ± 6 | 72 ± 6 | 67 ± 6 | 67 ± 6 |
| Men | 85.5% | 84.6% | 50.6% | 53.8% |
| Left ventricular ejection fraction | 50 ± 14 | 50 ± 14 | 54 ± 13 | 51 ± 14 |
| Diabetes mellitus | 31.3% | 35.8% | 24.4% | 29.3% |
| Hypertension | 78.7% | 79.8% | 68.4% | 72.6% |
| Smoking (%) | ||||
| Current | 7.2% | 5.4% | 8.2% | 6.7% |
| Past | 73.1% | 70.8% | 62.4% | 64.4% |
| Body mass index (kg/m 2 ) | 28.5 ± 7.9 | 28.4 ± 5.0 | 29.0 ± 6.5 | 28.8 ± 5.7 |
| Cholesterol (mg/dl) | ||||
| Total | 165 ± 40 | 167 ± 44 | 185 ± 45 | 179 ± 42 |
| Low-density lipoprotein | 94 ± 31 | 94 ± 34 | 107 ± 36 | 103 ± 35 |
| High-density lipoprotein | 40 ± 11 | 40 ± 12 | 46 ± 15 | 45 ± 14 |
| C-reactive protein (mg/100 ml) | ||||
| Median | 3.7 | 4.2 | 3.8 | 4.5 |
| Interquartile range | 2.1–8.5 | 2.3–9.0 | 2.5–8.5 | 2.7–8.9 |
| Narrowed coronary artery vessels (n) | ||||
| 1 | 10.9% | 9.7% | 6.6% | 9.3% |
| 2 | 21.7% | 20.0% | 21.8% | 21.8% |
| 3 | 67.4% | 70.3% | 71.6% | 68.9% |
| Aortic stenosis (%) | ||||
| None | 79.7% | 76.3% | 81.1% | 85.8% |
| Mild | 9.3% | 11.0% | 5.8% | 5.3% |
| Moderate to severe | 11.0% | 12.7% | 13.1% | 8.9% |
| Caucasian | ||||
| Maternal | 98.5% | 98.7% | 100.0% | 100.0% |
| Paternal | 98.3% | 98.9% | 100.0% | 99.7% |
| Moderate to severe aortic insufficiency | 6.4% | 5.2% | 5.2% | 8.5% |
| Moderate to severe mitral annular calcification | 8.5% | 12.5% | 9.2% | 8.6% |
| Moderate to severe mitral insufficiency | 19.1% | 18.8% | 24.8% | 28.7% |
| Characteristic | AS Group | Control Group |
|---|---|---|
| Age (years) | 73 ± 7 | 69 ± 6 |
| Men | 72.7% | 69.8% |
| Diabetes | 32.6% | 27.2% |
| Hypertension | 75.9% | 74.6% |
| Smoking | ||
| Current | 6.2% | 6.0% |
| Past | 65.1% | 66.3% |
| Cholesterol (mg/dl) | ||
| Total | 167 ± 42 | 175 ± 43 |
| Low-density lipoprotein | 96 ± 35 | 101 ± 34 |
| High-density lipoprotein | 43 ± 15 | 43 ± 14 |
| Aortic valve area (cm 2 ) | ||
| Median | 1.0 | NR |
| Interquartile range | 0.7–1.5 | |
| Aortic insufficiency ≥2+ | 13.6% | 3.8% |
| Mitral insufficiency ≥2+ | 25.4% | 23.9% |
| Indication for catheterization | ||
| Acute coronary syndrome | 26.4% | 29.1% |
| Congestive heart failure | 2.6% | 1.6% |
| Without valvular heart disease | ||
| Preoperative valvular heart disease | 57.1% | 8.6% |
| Stable coronary artery disease | 6.1% | 50.8% |
| Sudden cardiac death | 3.7% | 1.7% |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


