Chapter 4
Physiotherapy Modalities, Markers and Outcome Measures
Written by B.M. Morrow and H. van Aswegen
This chapter provides information about:
•Early mobilisation and graded exercise therapy.
•Cardiopulmonary physiotherapy techniques, their performance and contraindications and precautions to consider for adult and paediatric patients.
•Subjective and objective markers and outcome measures for the assessment of patient response to treatment interventions.
4.1. Early Mobilisation and Graded Exercise Therapy
‘Teach us to live that we may dread
unnecessary time in bed.
Get people up and we may save
our patients from an early grave.’
— RAJ Asher (1957)
People who have sustained traumatic injury are often subjected to a period of bed rest and immobility in the ward or intensive care unit (ICU) setting due to the nature and severity of their injury. The numerous complications of bed rest and immobility, affecting multiple organ systems, have been well described. Complications in both adults and children include muscle weakness and polyneuropathy, deep vein thrombosis, skin ulcers, disuse atrophy of the gut and significant psychological impact on both the patient and their family (Knight et al., 2009a,b,c; Fan, 2012; Dammeyer et al., 2013; Kocan and Lietz, 2013). These complications are exacerbated by inflammation, poor glycaemic control and drugs such as neuromuscular blocking agents administered to the patient (Gosselink et al., 2011). Complications specific to the respiratory system include pneumonia, positional atelectasis, secretion retention, airway obstruction and decreased lung volumes (Knight et al., 2009a; Truong et al., 2009). Survivors of critical illness or injury complain of weakness for months and sometimes even years after discharge from hospital (Fan, 2012).
The general aims of physiotherapy in people following trauma are summarised in Table 4.1 and should be considered alongside the limitations that their injuries will present.
In order to achieve these goals of therapy, physiotherapists must remain cognisant about treating every patient holistically. Specific considerations and applications according to injury type are presented in the relevant chapters of this book.
Table 4.1:General aims of physiotherapy in people who sustained traumatic injury.
•To decrease pain associated with joint stiffness and muscle spasm •To rehabilitate the patient to the highest level of functional independence as possible •To restore mobility, normal gait pattern and bodily posture within the limitations posed by each type of injury •To improve muscle strength •To maintain or restore full joint range of motion and muscle length •To facilitate early weaning from mechanical ventilation for those who are intubated and ventilated •To improve breathing pattern and reduce the work of breathing •To ensure adequate ventilation of all areas of the lungs •To assist in the removal of excessive bronchial secretions •To prevent or resolve pulmonary complications |
4.1.1. Early mobilisation
During the acute and sub-acute stages after a traumatic injury, the emphasis of physiotherapy management should be on the mobilisation of the patient as soon as their condition has stabilised, appropriate to the:
•age and developmental level of the patient;
•cognitive function of the patient;
•patient’s acuity of illness;
•patient’s general condition; and
•type, severity and stability of the injury.
Physical activities that may be used even for cooperative intubated and ventilated patients include (Gosselink et al., 2011; Fan, 2012; Stiller, 2013):
•active limb exercises;
•functional bed exercises;
•sitting in bed or in a chair;
•standing and transferring from bed to chair; and
•marching on the spot or walking away from the bedside, using an assistive device if indicated.
The aims of mobilisation include improving thoracic mobility; increasing lung volumes (Zafiropoulos et al., 2004) and functional residual capacity (FRC); improving functional activity, exercise tolerance, muscle strength and cardiovascular fitness (Stiller, 2013), which aid in weaning from mechanical ventilation; preventing postural deformities that could impact on function (including respiratory function); improving bone ossification; improving bladder and bowel function; and providing psychological benefits to the patient (Bailey et al., 2007; Truong et al., 2009).
In adults, mobilisation has been shown to be safe, feasible and effective in the early stage of ICU admission (Bailey et al., 2007; Morris et al., 2008; Schweickert et al., 2009; Truong et al., 2009; Stiller, 2013). Benefits of early rehabilitation include reducing hospital and ICU length of stay, increasing ventilator free days, improving muscle strength and health-related quality of life (QOL) (Kayambu et al., 2013; Stiller, 2013). Mobilising any patient in the ICU requires a team approach in order to ensure safe and effective graded mobilisation (Dammeyer et al., 2013; Kocan and Lietz, 2013). Note that this area has not been well studied in the paediatric population.
4.1.2. Exercise therapy
Exercise therapy is associated with a multitude of beneficial effects on mental and physical health.
4.1.2.1. Mental health benefits
Plato stated in the fourth century BC that God provided man with two means in order to lead a successful life: namely education and physical activity. One is for the soul and the other for the body and they are meant to be used together for man to attain perfection (Ströhle, 2009). Physical activity has been shown to have beneficial effects on mood and anxiety levels, life satisfaction, feelings of well-being and cognitive functioning (ACSM, 2014), whereas physical inactivity may be associated with the development of mental disorders such as depression and anxiety (Ströhle, 2009).
4.1.2.2. Physical health benefits
Physical fitness involves the cardiovascular, respiratory and musculoskeletal systems. The cardiovascular and respiratory systems are conditioned through aerobic exercise, whereas the musculoskeletal system is conditioned through aerobic, resistance and flexibility exercises (Warburton et al., 2006). Aerobic exercise has various beneficial effects on the cardiopulmonary system and these include decreased blood pressure, increased stroke volume with a resultant decrease in resting heart rate, increased synthesis of high-density lipoproteins and improved insulin sensitivity (ACSM, 2014). As a result of aerobic exercise, muscles develop new capillaries, which increase oxygen extraction ratio. More skeletal muscle cell mitochondria are also produced and therefore maximal oxygen uptake improves and minute ventilation decreases as physical fitness improves. As maximal oxygen uptake increases, less energy is utilised to perform activities of daily living (ADL) and as a result perceived level of QOL improves (ACSM, 2014).
Skeletal muscle mass, strength and aerobic and anaerobic capacity increase with resistance exercise (Kirstensen and Franklyn-Miller, 2012). Several factors influence muscle strength, such as neural control, muscle cross-sectional area and muscle length. Neural control includes the number of motor units that are recruited during a muscle contraction as well as the rate at which the motor units are stimulated (ACSM, 2009; Kirstensen and Franklyn-Miller, 2012). During the first few weeks of resistance training, the brain learns to extract more force from a specific amount of contractile muscle fibres. Muscle cross-sectional area determines the force with which a muscle contracts, whereas maintenance of resting muscle length is important to ensure the greatest strength being generated through that muscle, as actin and myosin are in an optimal position for cross-bridge links to form during muscle contraction (Harman, 2000; ACSM, 2009). New bone formation is stimulated through weight-bearing activities that exceed the minimal essential strain of bone and enhance the osteoblast activity that stimulates bone growth. Examples of such activities are walking, stair climbing and running (Conroy and Earle, 2000; ACSM, 2014).
Recently evidence has come to light that shows that low-level fitness is associated with increased risk of cardiovascular and non-cardiovascular disease mortality for both men and women across short- and long-term follow up periods (Vigen et al., 2012). This information underscores the importance of exercise and its health benefits for all persons, but especially for those recovering from ill health.
4.1.2.3. Exercise prescription
Exercise prescription for patients with traumatic injury should follow the same principles as that used for healthy individuals — namely frequency, intensity, time and type (ACSM, 2014) — together with consideration for progression of exercise therapy. Components of a single exercise session are:
•warm up;
•conditioning (aerobic and resistance exercises);
•cool down; and
•stretching and flexibility exercises.
No guidelines for exercise prescription for patients in the acute or subacute phases after traumatic injury have been identified in the literature. The recommendations provided in Table 4.2 are based on exercise prescription guidelines for deconditioned adult patients with critical illness or untrained individuals (ACSM, 2009; Hanekom et al., 2011; Kirstensen and Franklyn-Miller, 2012; ACSM, 2014).
| Principles of exercise prescription | Recommendations |
| Frequency | Start with once per day; progress to twice daily or more frequently as indicated |
| Intensity | Aerobic exercise: Resistance exercise: •Low load starting with 45–50% of one repetition maximum (RM); progress to 50–70% of one RM •Repetitions and volume: start with one set of 8–12 repetitions; progress to three sets of 8–12 repetitions before the load is increased •Rest periods: 1–2 minutes between exercise |
| Type | Aerobic exercise: •Bedside cycle ergometer •Walking, step climbing, stationary bicycle, treadmill walking or running Resistance exercise: •Body weight, free weights, resistance bands, weight machines •Concentric, eccentric and isometric exercises of the trunk, upper and lower limbs •Uni- and bilateral limb exercises •Single- and multiple-joint exercises |
| Time | Aerobic exercise: •Start with 5–10 minutes; progress to 20 minutes |
The study by Berney et al. (2012) demonstrated a unique approach to the rehabilitation of survivors of critical illness from ICU admission through to exercise provided for these patients in an outpatient setting. Patients with central nervous system involvement or unstable fractures were excluded from this study. The exercise prescription format that these researchers used with their patients in the ICU and ward setting is summarised in Table 4.3. The progression of aerobic exercise and resistance training was done according to each patient’s re-assessment findings (Berney et al., 2012).
| ICU | Ward environment | |
| Duration of exercise (aerobic, resistance training, functional retraining) | 15 minutes to complete: •March on the spot* OR •March on the spot and strength OR •March on the spot and strength and functional retraining OR •Whole body bed exercises | 10–15 minutes |
| Frequency of exercise | Two 15-minute sessions per day; progressed to one 30-minute session per day | Two 30-minute sessions per day; progressed to one 60-minute session per day |
| Intensity of exercise | ||
•Aerobic exercise | •Target rate of perceived exertion (RPE) using modified Borg scale 3–5 (moderate to severe exertion) | •Interval training for endurance RPE 4–5 or 60–70% HRmax; after two weeks RPE increased to 5–6 |
•Resistance training | •Until fatigue
| •75% of five RM
|
| Type of exercise | ||
•Aerobic exercise | •Marching on the spot; progressed to walking away from bedside | •Walking on ward or on treadmill; progressed to stationary bicycle |
•Resistance training | •Active to resisted for those with > Grade 3 muscle strength; active-assisted to active for those with < Grade 3 strength | •Resisted limb, pelvis and trunk exercises using own body weight, dumbbells or resistance bands |
•Functional retraining | •Sit-to-stand, rolling, supine to sitting, trunk control or balance | •Same activities as in ICU and progressed to stair climbing |
*March on spot = three repetitions of 70% of initial physical function in ICU test (PFIT) duration.
The information summarised in Table 4.3 serves as a guide for exercise prescription in the acute care setting and should be modified according to each patient’s ability and type of traumatic injury.
No published exercise prescription guidelines for in-hospital rehabilitation could be found for children or adolescents recovering from traumatic injuries or critical illness. In the absence of formal guidelines, clinicians use safe and appropriate fun activities, such as kicking a light ball while sitting in a chair, reaching up to pop bubbles or playing ‘Simon Says’ in bed or in a chair while carefully monitoring the child’s response to these activities. Progression of the duration and intensity of these exercises is performed when appropriate.
We hope that the guidelines shared in this section will provide physiotherapists with a framework within which to tailor exercise prescription in the acute care setting for individual adult and paediatric patients with traumatic injuries based on their pre-injury health status, level of physical fitness and physical ability. The physiotherapist is a member of the interprofessional team that cares for patients with traumatic injury in the acute care setting; therefore decision making regarding the progression of exercise therapy should be done jointly with the team.
Quality of life, exercise therapy and rehabilitation of trauma survivors in the chronic stage following injury (after discharge from hospital) are discussed in Chapter 10.
4.2. Cardiopulmonary Physiotherapy Techniques
A patient who suffered traumatic injury may undergo prolonged periods of immobility due to the severity of injuries sustained, which places them at increased risk for the development of pulmonary complications. Chest physiotherapy, in the form of breathing and exercises, for postoperative and trauma patients was first described in the early 1900s (MacMahon, 1915). Today the term ‘chest physiotherapy’ or ‘respiratory physiotherapy’ refers to a large variety of techniques used by cardiopulmonary physiotherapists to prevent the onset of pulmonary complications or manage existing pulmonary complications that have developed in the patients under their care. Despite the widespread use of chest physiotherapy in clinical practice, research evidence to its use remains inconclusive (Stiller, 2013).
Physiotherapists often use multiple techniques in a single treatment session. In the management of critically ill patients it is recommended that routine multimodality chest physiotherapy is no longer appropriate and treatment should be patient-centred and planned based upon careful, thorough clinical and radiographical individual patient assessment and a favourable risk–benefit ratio (Woodward and Jones, 2002; Stiller, 2013). The same approach to patient care for those with traumatic injury is therefore recommended. The frequency of chest physiotherapy treatment should be determined by the needs of each individual patient. Appropriate administration of analgesia is essential to ensure adequate lung ventilation and secretion clearance (Ridley and Heinl-Green, 2002). The precise role of the physiotherapist in managing patients with traumatic injuries is likely to differ among different settings, according to the country, local tradition, staffing levels, training and levels of expertise available.
4.2.1. Breathing exercises
In the early 1900s, MacMahon (1915) described using upper extremity exercise, lateral costal expansion exercises and localised thoracic expansion techniques in an attempt to re-expand areas of atelectasis. These techniques are used to this day, in addition to other types of breathing exercises, particularly in non-ventilated patients and those able to obey commands and cooperate voluntarily.
4.2.1.1. Localised thoracic expansion exercises
4.2.1.1.1. How to perform the technique
This technique can be performed in adults and children, with the patient positioned either lying or sitting. The therapist’s hands are placed over the area of decreased thoracic expansion, providing tactile stimulus, end-expiratory stretch and resistance to the inspiratory muscles (Kigin, 1981). If the patient is alert, verbal commands and encouragement to inhale deeply should be given to ensure optimal thoracic expansion is achieved in the specific area. There is still a lack of standardisation in the technique of performing this type of breathing exercise.
4.2.1.1.2. Contraindications and precautions
•End-expiratory stretch should not be used over the thorax in the presence of rib fractures.
•Pain due to surgical incisions that involve the abdomen or thorax and around insertion sites of intercostal drains will require adaptation of the intensity of an end-expiratory stretch.
4.2.1.2. Active cycle of breathing technique
The active cycle of breathing technique (ACBT) is a combination of breathing techniques that were developed for patients with chronic lung diseases such as cystic fibrosis, but have since been adopted by many physiotherapists for patients with a wide range of conditions resulting in secretion retention and lung volume loss. It can be used in children (from as young as three years old) able to understand and follow commands, as well as cognitively intact adults.
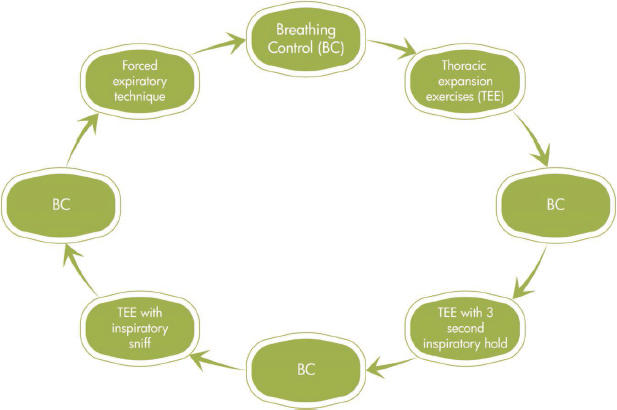
The ACBT consists of a cycle of breathing control (diaphragmatic breathing) and thoracic expansion exercises (deep lateral basal breaths), with or without inspiratory holds or inspiratory sniffs, followed by one or two forced expiratory techniques (FET), also known as ‘huffs’; FET should always be followed by breathing control to prevent airway or alveolar collapse due to the forced expiration (Lewis et al., 2012) (see Fig. 4.1).
The sequence in which these components are performed may vary in order and number, but all the components must be used during a treatment session (Lewis et al., 2012). The sequence is determined by the needs of each patient, as more emphasis might be placed on thoracic expansion exercises (TEE) in the case of lung volume loss, with less focus on FET.
4.2.1.2.1. How to perform the technique
The patient can perform ACBT in any comfortable position. The effectiveness with which a patient performs ACBT is dependent on the quality of instruction received from the physiotherapist. Table 4.4 summarises the instructions that may be given to patients when performing each component of ACBT. The physiotherapist should ensure that the patient maintains a steady respiratory rate while performing breathing control (BC) and TEE. The physiotherapist should use their voice to encourage the patient, especially during the TEE and FET components of ACBT.
Fig. 4.1:An example of the active cycle of breathing technique. The sequence can be adjusted to place emphasis on a particular component according to the needs of each patient.
Table 4.4:Instructions related to each component of ACBT.
| Component | Instructions to the patient |
| Breathing control | •Breathing control is performed during relaxed normal (tidal volume) breathing. The patient is instructed to place two fingers directly below the breast bone (xiphisternum) and to feel the diaphragm rise and fall during relaxed breathing. Breathing control is the ‘recovery phase’ of ACBT. |
| Thoracic expansion exercises: | |
•Basic TEE | •Thoracic expansion exercises are performed during the inspiratory phase of deep breathing. The physiotherapist’s or patient’s hands should be placed over the bottom part of the ribcage on the sides of the chest wall (lateral basal lung segments). The patient is instructed to ‘breathe into the hands’ during deep inspiration. |
•TEE with three-second inspiratory hold | •The same technique is used as described for basic TEE. The patient is instructed to ‘hold’ the inspiratory breath for three seconds before exhaling. The breath hold aims to improve airflow distribution through the collateral ventilation pathways at bronchiole-alveolar level. |
•TEE with inspiratory sniff | •The same technique is used as described for basic TEE. The patient is instructed to breathe in as deeply as possible and then to ‘hold’ the breath. Immediately after the ‘hold’ the patient is instructed to sniff in an additional breath through the nose and to then only exhale. |
| Forced expiratory techniques: | |
•Long FET (low volume FET) | •The patient relaxes their arms by the side of the body and is instructed to perform one or two long huffs. This should be done with an open glottis. Huffing is similar to the method used for misting up a mirror or a pair of reading glasses to clean them. Long FET aims to recruit secretions from the periphery of the lungs and move them more centrally for evacuation from the airways. |
•Short FET (high volume FET) | •The same technique is used as described for long FET; however, the patient is instructed to perform one or two short, sharp huffs at high lung volume with an open glottis. Short FET assists with evacuating secretions from the central airways. |
4.2.1.2.2. Contraindications and precautions
•There are no contraindications or precautions to using ACBT on awake and cooperative patients with traumatic injury.
•It is important to instruct the patient to breathe away from the physiotherapist’s face while performing ACBT for infection control purposes.
•It is important to note that in the presence of pain, general weakness and dyspnoea, these exercises may only be effective if the patient sits with back support (chair or semi-Fowlers position in bed).
Active cycle of breathing technique has been found to be comparable to other airway clearance techniques (conventional chest physiotherapy and oscillatory expiratory pressure devices) for short-term improvements in secretion clearance (Lewis et al., 2012). Forced expiratory technique, specifically, may be a useful technique to clear secretions in patients following thoracic or abdominal injury, where coughing may be extremely painful.
4.2.1.3. Breath-stacking
Breath-stacking was described in the late 1980s by Marini and colleagues (1986) as an alternative method to facilitate deep breathing and increase vital capacity, hence cough effectiveness, in uncooperative patients. This breathing technique has gained popularity over the years and is used in many hospitals by cardiopulmonary physiotherapists as part of patient care.
4.2.1.3.1. How to perform the technique
The patient should be placed in a comfortable position. A silicon mask with a one-way valve is placed over the patient’s face. The valve should be set to allow only inspiration (the expiration branch should be closed) and the patient should be encouraged to perform successive inspiratory efforts over a 20-second period; the result being stacking of breaths on top of each other to increase lung volumes. After the 20-second period the exhalation branch is opened to allow the patient to exhale freely (Dias et al., 2008).
4.2.1.3.2. Contraindications and precautions
No adverse effects have been reported in the literature as a result of the use of breath-stacking.
The effectiveness of breath-stacking compared to incentive spirometry in the physiotherapy management of patients after upper abdominal surgery was investigated by Dias and colleagues. These authors reported the superiority of breath-stacking over incentive spirometry in relation to inspired lung volume in the postoperative period, with more pronounced lung volume reductions in patients who were treated with incentive spirometry (Dias et al., 2008).
4.2.1.4. Glossopharyngeal breathing
This is a useful breathing technique for patients following spinal cord injury with paralysis or weakness of the respiratory muscles. The inability to take a deep inspiration is one of the fundamental components of a cough, which is missing in tetraplegic patients. Glossopharyngeal breathing can also be used to maintain or improve lung and chest wall compliance (Pryor et al., 2008).
4.2.1.4.1. How to perform the technique
4.2.1.4.2. Contraindications and precautions
•Glossopharyngeal breathing is contraindicated in patients with chronic obstructive pulmonary disease, as the positive pressure could exacerbate air trapping in the lungs (Pryor et al., 2008).
•A fall in blood pressure can occur due to the build-up of intrathoracic pressure and therefore the technique is contraindicated in patients with cardiovascular instability or cardiac dysfunction (Pryor et al., 2008).
•Training sessions should be kept short initially, as learning the technique can be tiring.
4.2.1.5. Respiratory muscle training
Respiratory muscle training (RMT) may be useful where there is the potential to strengthen the inspiratory or expiratory respiratory muscles, which might have become weak due to direct or indirect injury and/or prolonged mechanical ventilation. Training of the inspiratory muscles of patients who undergo prolonged mechanical ventilation is effective in reducing the duration of ventilation (Cader et al., 2010). Inspiratory muscle training can be achieved through the adjustment of trigger sensitivity or pressure support settings on the mechanical ventilator or with the use of a spring-loaded resistance device (Caruso et al., 2005; Cader et al., 2010; Martin et al., 2011). A variety of suppliers make inspiratory and expiratory muscle-trainer (IMT/EMT) devices for use in the clinical setting, such as the Threshold® IMT or EMT devices (supplied by Respironics) and POWERbreathe®. These threshold devices are made of plastic, spring-loaded and calibrated (Fig. 4.2). The spring applies resistance to either inspiratory or expiratory breaths. In some countries electronic respiratory muscle training devices may be available.
4.2.1.5.1. How to perform the technique
4.2.1.5.1.1. Spring-loaded device. The patient should be placed in a supported 45° seated position in bed or upright in a chair. To establish baseline inspiratory muscle strength, three measurements of the patient’s maximal inspiratory pressure (MIP) should be made using a pressure manometer. The three readings should not vary by more than 20% and the best of the three readings is recorded (ATS/ERS, 2002). The device is attached directly to the endotracheal or tracheostomy tube using a T-piece A-connector. Oxygen therapy can be supplied at the same time through an additional connector attached to the trainer device, if indicated. The patient would need to be able to breathe without support from the ventilator for a few minutes in order to be trained with this device. Spontaneously breathing patients can use this device with a mouthpiece. There are no uniform recommendations in the rehabilitation literature for training with spring-loaded IMT devices. Examples of strategies used by various researchers are summarised in Table 4.5.
Table 4.5:Examples of IMT strategies using spring-loaded devices in patients who are intubated and ventilated*.
| Percentage of maximal inspiratory pressure | Highest pressure tolerated | |
| Cader et al. (2010) | Condessa et al. (2013) | Martin et al. (2011) |
•Set the spring in the IMT device to 30% of the patient’s MIP •The patient breathes through the device for five minutes twice daily •Inspiratory resistance is increased by 10% of baseline MIP daily as tolerated | •Set the resistance in the device to 40% of MIP •The patient performs five sets of 10 breaths, twice daily, for seven days per week | •Set the resistance in the device to the highest pressure level that the patient can tolerate •The patient performs four sets of six to 10 breaths five days per week |
*The majority of subjects in all three studies had medical or surgical admission diagnoses.
The optimal dosage of training has not yet been established.
4.2.1.5.1.3. Adjustment of pressure support setting on a mechanical ventilator. Another method of IMT used by physiotherapy clinicians is the temporary adjustment of pressure support while the patient performs deep breathing, observing the ventilator screen and attempting to increase the inspiratory tidal volume values with each deep breath (ventilator biofeedback). Pressure support provides inspiratory assistance to the patient during the inspiratory phase of breathing and in so doing unloads the inspiratory muscles. Therefore, the reduction of pressure support imposes resistance to inspiration and greater inspiratory muscle activity. Pressure support may be reduced with 2–3 cm H2O in a treatment session while the patient performs deep breathing with ventilator biofeedback. The patient should be closely observed for signs of fatigue. Care must be taken to reset the pressure support to the pre-treatment level at the end of each treatment session. Research evidence to support the effectiveness of this type of IMT could not be found. Again, this type of intervention needs to be discussed with the medical or surgical team looking after the patient, as explained above.
4.2.1.5.2. Contraindications and precautions
•A precaution to RMT is that the intubated patient should not be pushed to the point of respiratory muscle exhaustion during training. This delays the process of weaning from mechanical ventilation.
•Patients should always be pre-oxygenated prior to performing each set of IMT repetitions.
A systematic review and meta-analysis of 11 studies of RMT in 212 participants with cervical spine injury concluded that RMT did appear to be effective for increasing respiratory muscle strength and possibly lung volumes for this population group (Berlowitz and Tamplin, 2013). There were insufficient data to report on the effect of RMT on functional outcomes like dyspnoea, cough efficacy, respiratory complications, hospital admissions and health-related QOL.
There are currently no studies of RMT in the paediatric post-surgical, ICU or trauma populations.
4.2.2. Devices
4.2.2.1. Incentive spirometry
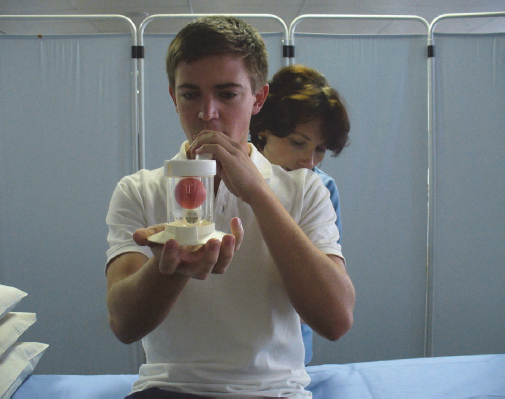
Incentive spirometry is a method used to encourage deep breathing and mimic a sigh, and is used in postoperative patients (Branson, 2013). Incentive spirometry provides visual feedback in terms of volume attained during a deep breath and might therefore be a useful adjunct to deep breathing exercises (Agostini and Singh, 2009). Flow- and volume-oriented spirometers are available, including spirometers designed specifically with children in mind. Volume spirometers require less effort and lead to more laminar airflow, while flow spirometers can be difficult for the older and weaker patients, as greater muscle work is required to lift up the first ball. This also leads to a turbulent flow which does not penetrate as deeply into the airways as laminar flow.
4.2.2.1.1. How to perform the technique
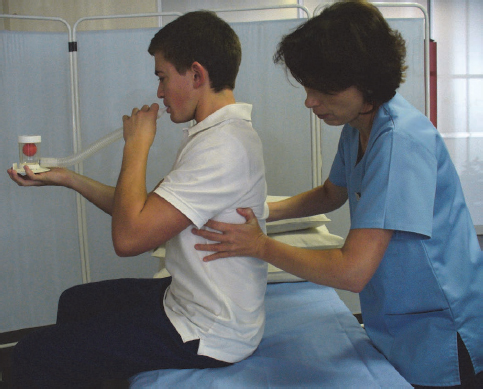
The patient should be in a supported high-sitting position in bed or seated in an upright position in a chair while holding the incentive spirometer parallel with the floor. Shoulder girdle relaxation exercises should be performed prior to initiating incentive spirometry. The physiotherapist should place their hands on the lateral basal aspects of the patient’s thoracic cage (Fig. 4.3) and instruct the patient to take in a long, slow breath while attempting to lift the ball or balls in the chamber of the spirometer. During deep inspiration the patient must attempt to breathe into the physiotherapist’s hands in order to facilitate expansion of the basal lung segments. The physiotherapist must ensure that the patient achieves expansion in the basal lung regions without elevation of the shoulder girdle (Fig. 4.4). If the patient manages to perform the technique correctly, with adequate basal lung segment expansion, the volume of the incentive spirometer should be adjusted accordingly, starting at the lowest and progressing to the highest volume.
Fig. 4.3:Lateral basal lung segment expansion combined with slow, deep inspiration through the incentive spirometer.
Fig. 4.4:Shoulder girdle relaxation is encouraged while performing deep inspiration with lateral basal lung segment expansion using the incentive spirometer.
As the patient masters the technique under the guidance and supervision of the physiotherapist, they should be encouraged to continue using incentive spirometry on an hourly basis during the day. The physiotherapist should regularly review the patient’s technique and changes in their condition in order to provide adequate instruction for the progression of treatment until the desired lung volumes are achieved.
4.2.2.1.2. Contraindications and precautions
•There are no contraindications or precautions to the use of incentive spirometry in awake, cooperative and spontaneously breathing patients with traumatic injury.
•The physiotherapist should stand to the side of or behind the patient during treatment for infection control purposes to prevent the patient from coughing over them.
•Extremely weak trauma patients might become discouraged if they cannot master incentive spirometry due to their weakness. In such cases alternative methods should be considered.
4.2.2.2. Intermittent positive pressure breathing
Intermittent positive pressure breathing (IPPB) refers to the delivery of patient-triggered positive airway pressure throughout inspiration, with airway pressure returning to atmospheric levels during expiration. It is used in the management of spontaneously breathing patients and performed using a positive pressure device such as the Bird Mark 7 or Mark 8 ventilator (Pryor et al., 2008). Patients who suffer from increased work of breathing due to respiratory muscle fatigue or weakness as well as those who breathe at low lung volumes due to postoperative pain find relief of symptoms when using IPPB. By augmenting tidal volume and reducing the work of breathing, IPPB may enhance secretion clearance and re-expand collapsed areas of the lung. Work of breathing is, however, only reduced if the patient relaxes completely and does not ‘assist’ the machine (Pryor et al., 2008).
4.2.2.2.1. How to perform the technique
The patient is placed in a relaxed supported sitting or side lying position as indicated. A gravity-assisted position may also be used if the patient is not too breathless. The IPPB machine is connected to the oxygen outlet on the wall of the hospital ward or ICU. The breathing circuit is attached to the Bird ventilator and a mouth piece or tight-fitting face mask may be attached to the patient side of the circuit. The IPPB machine delivers dry oxygen to the patient and therefore humidification of the airways is important. Ensure the nebuliser in the circuit is filled with four ml of saline or a mucolytic or bronchodilator drug, if indicated. Auscultate the patient’s chest to identify areas of decreased breath sounds.
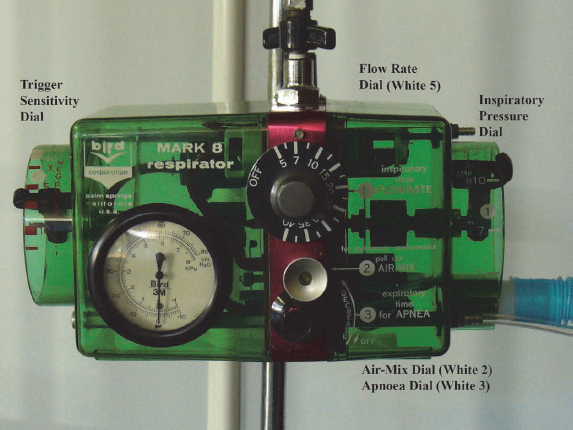
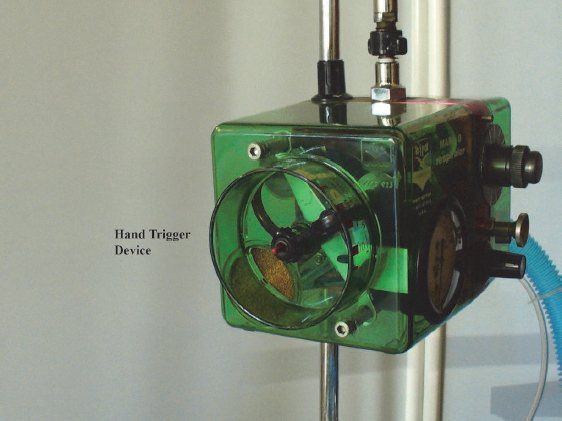
Some physiotherapists may find using the IPPB machine a daunting task due to all the dials on the machine (Figs 4.5A and B); however, with practice, the use of IPPB becomes second nature.
Table 4.6 summarises the steps to follow when setting up the IPPB machine for patient treatment.
Throughout treatment the patient is instructed not to block the flow of air through the circuit with their tongue, but to allow the machine to inflate their lungs. On expiration the machine stops cycling to allow the patient to exhale fully. The duration of treatment is dependent on the time taken to deliver the medication in the nebuliser to the patient. Intermittent positive pressure breathing can be combined with expiratory chest wall vibrations and gravity-assisted or modified gravity-assisted positioning to further enhance expiratory flow and improve the clearance of retained secretions.
Table 4.6:General steps to follow in setting up an IPPB machine for patient treatment and guidance for progression of management.
| Dials on IPPB machine | Initial settings | Progression of settings |
| Air mix control | The control should be pulled outwards to its fullest length to ensure that a mixture of approximately 45% oxygen is delivered to the patient. If the air mix control is pushed back to its shortest length, it delivers approximately 100% oxygen, which is inappropriate for physiotherapy patient care. | Air mix control remains unchanged at 45% oxygen. |
| Sensitivity or ‘trigger’ | The patient needs to generate a negative flow (sucking pressure) with minimal effort through the circuit to trigger the machine to deliver positive pressure during the remainder of the inspiratory cycle. The sensitivity dial should be set to a low value (usually −5 to −7 cmH2O) and should be adjusted for each individual patient. | If strengthening of the respiratory muscles becomes an aim of treatment, the sensitivity dial can be adjusted to a higher number so that the patient works harder to generate negative flow through the circuit at the start of inspiration. |
| Flow rate | This dial regulates the rate of flow of inspiratory gas into the patient’s airways. The lower the flow rate, the deeper the gas penetrates down the tracheobronchial tree; therefore the patient takes a long slow breath. The breathless patient may initially not manage to breathe at low flow rates and thus a higher flow rate (15–20 L/min) is chosen to match the patient’s own respiratory rate. | As the patient settles on the IPPB machine, flow rate can be gradually adjusted to a lower setting (5–7 L/min) to allow for longer inspiration in order to re-expand collapsed airways and assist with the mobilisation of secretions. |
| Inspiratory pressure | The Bird ventilator delivers a range of positive pressure from 0 cmH2O to 40 cmH2O. An inspiratory-positive pressure of 10 cmH2O is initially selected for treatment. The pressure gauge on the front of the IPPB machine indicates whether the desired pressure is being delivered to the patient. After the patient has breathed at this level of pressure for a few minutes, re-auscultate the chest to assess if airflow has increased in areas of the lungs that initially presented with decreased breath sounds. | If the changes in breath sounds are minimal, increase the inspiratory pressure gradually by 2–3 cmH2O until a level of pressure is reached that results in improved breath sounds. |
| Hand-triggering device | This device should be used to test that there are no leaks in the circuit before patient treatment is initiated. It is also used to stop the device from cycling at any time during treatment, if indicated. | Not applicable. |
| Apnoea dial | Must be switched off, as during physiotherapy treatment the IPPB machine is not used as a ventilator. | |
| Expiratory time dial | Must be switched off, as during physiotherapy treatment the IPPB machine is not used as a ventilator. |
4.2.2.2.2. Contraindications and precautions
•Undrained pneumo- or haemothorax (Pryor et al., 2008).
•Active tuberculosis as it may contaminate the equipment.
•Large bullae in patients with chronic obstructive pulmonary disease, as positive pressure may lead to rupture of the bullae and result in a pneumothorax (Pryor et al., 2008).
•Lung abscesses, as positive pressure may lead to an increase in the size of the abscess (Pryor et al., 2008).
•Haemoptysis or pulmonary haemorrhage until the bleeding has stopped. Always discuss with the medical team under these conditions.
•Presence of a bronchial tumour in the proximal airways (Pryor et al., 2008). On inspiration, air may flow around the tumour into the airways due to the natural expansion of the diameter of the airways by contraction of the elastin and collagen fibres attached to the outside of the airways. Flow of air out of the airways may be prohibited by the tumour during expiration as airway diameter reduces (relaxation of elastin and collagen fibres) and there is risk of the development of a ‘ball valve’ effect and air trapping.
•Before use in patients who had recent thoracic surgery, discuss the appropriateness of IPPB therapy with the surgical team.
•Reduction in cardiac output may result from IPPB due to the increased intrathoracic pressures and therefore IPPB should be used with care in haemodynamically unstable patients.
•Intermittent positive pressure breathing is not indicated for the treatment of young children due to the potential danger of pneumothorax. There are insufficient safety studies to support using this technique in this vulnerable population. If the physiotherapist deems treatment with IPPB to be necessary, it should only be used in older, cooperative children and maximal inspiratory pressures should be kept lower than or equal to 30 cmH2O.
There is currently little research evidence for the use of IPPB in patients with traumatic injuries, although it remains a frequently used adjunct to physiotherapy treatment in some clinical settings.
4.2.2.3. Mechanical insufflation-exsufflation
Mechanical insufflation-exsufflation is the delivery of positive and negative pressure to a patient’s airways through an insufflator-exsufflator machine (CoughAssist In-exsufflator® or Nippy Clearway®) in order to augment a weak or absent cough. Positive pressure is delivered to the airways during inspiration to augment lung volumes and the sudden switch to negative pressure initiates expiration and augments peak cough expiratory flow (Pryor et al., 2008; Morrow et al., 2013).
4.2.2.3.1. How to perform the technique
The patient should be seated in a comfortable position. Non-invasive patient administration of mechanical insufflation-exsufflation can be achieved using a tight-fitting face mask or mouth piece; alternatively, the machine may be attached to an artificial airway such as an endotracheal or tracheostomy tube. The delivery of mechanical insufflation-exsufflation through a face mask or mouth piece involves the patient’s active participation to augment their weak, ineffective cough through partially closing the glottis. If glottis control is totally absent in a spontaneously breathing patient, therapy with this device will be ineffective (Toussaint, 2011). The delivery of mechanical insufflation-exsufflation through an artificial airway is effective even in the absence of a patient’s spontaneous cough or glottis control (Toussaint, 2011). It should be noted that laboratory data suggests that the inner diameter of the artificial airway reduces peak expiratory flow during mechanical insufflation-exsufflation therapy and therefore cough effectiveness; the narrower the diameter, the lower the peak flow for a given expiratory pressure (Guérin et al., 2011). These findings remain to be verified in in vivo studies.
It is recommended that inspiratory positive pressure be set at 15–20 cmH2O or at a level that is comfortable for the patient during the initial treatment session (Pryor et al., 2008; Bott et al., 2009), and that the negative expiratory pressure should be set at the same value. Treatment progression, if suitable for the individual patient, involves a gradual increase in positive pressure level to achieve a negative pressure level that is 10–20 cmH2O higher than the positive pressure level (Pryor et al., 2008). Treatment pressures as high as +40 cm H2O and −40 to −45 cmH2O have been reported in all age groups. Some clinicians measure the patient’s MIP and then calibrate the inspiratory and expiratory pressure settings as a percentage of MIP instead of using pre-determined set pressures as mentioned above; however, evidence to support this method of calibration could not be sourced.
Ventilator-induced lung injury is a well-known topic of discussion in critical care literature. The use of low inspiratory tidal volumes, maintenance of positive end-expiratory pressure (PEEP) and of wide-pressure swings are recommended to protect the lungs from injury (Carpenter, 2004; Fuller et al., 2013; Park et al., 2013). The high pressures delivered during mechanical insufflation-exsufflation, therefore, raise concern about the long-term effects that this therapy might have on lung mechanics and patient outcomes (Morrow et al., 2013). Lower comfortable pressures may be more beneficial and safer, particularly in the paediatric population.
Patients with spinal cord injury, neuromuscular disease or thoracic cage deformity probably derive the greatest benefits from mechanical insufflation-exsufflation in relation to improved peak cough expiratory flow and maintenance of lung volumes (Morrow et al., 2013). The combination of mechanical exsufflation with manual chest shaking and vibrations and suctioning further enhances secretion clearance (Anderson et al., 2005; Finder, 2010). Patients tend to prefer treatment with mechanical insufflation-exsufflation over airway suction alone, as the device assists with the mobilisation of secretions into the central airways, which are easily and effectively cleared with superficial suction instead of deep airway suction (Schmitt et al., 2007; Toussaint, 2011).
4.2.2.3.2. Contraindications and precautions
•Mechanical insufflation-exsufflation should not be used in the presence of an undrained pneumothorax, haemothorax or pleural effusion, fresh haemoptysis or cardiovascular system instability.
•In addition, the physiotherapist should monitor the patient closely for the development of complications such as abdominal distension, gastro-oesophageal reflux, pneumothorax, cardiac arrhythmia and changes in mean arterial pressure (MAP). If these complications arise, appropriate assistance should be sought from the other members of the interprofessional team (Morrow et al., 2013).
•Active tuberculosis, due to contamination of the equipment.
•In children, pressures should be carefully increased from an initial low level, with observation and monitoring of clinical response, in order to prevent barotrauma. Pressures should be kept at the lowest level possible that is effective for clearing secretions.
•Do not perform mechanical insufflation-exsufflation therapy within 30 minutes of the patient’s last meal.
The effects of mechanical insufflation-exsufflation on outcomes of patients in the ICU have not been studied (Gosselink et al., 2011).
4.2.2.4. Positive expiratory pressure therapy
Positive expiratory pressure (PEP) therapy is believed to improve secretion clearance by increasing gaseous pressure behind the secretions using the collateral ventilation channels in the lung periphery or by preventing airway collapse during exhalation (McCool and Rosen, 2006). It is reasonable to assume that PEP therapy improves patient oxygenation, as FRC increases when partially collapsed peripheral airways are recruited through collateral ventilation pathways, with a resultant enlargement of the surface area available for gas exchange. Most studies of PEP therapy have been done in adults and children with chronic lung disease, particularly cystic fibrosis. Apparatus for PEP therapy includes a mask, one-way valve and expiratory resistors (PEP resistors) for continuous PEP; alternatively, bubble PEP (underwater PEP or ‘blow bottles’) or flutter devices for oscillating PEP. ‘Fun’ PEP devices and toys (e.g. windmills or bubbles), providing uncontrolled PEP levels, can also be used with children to optimise compliance.
4.2.2.4.1. How to perform the technique
The patient should be placed in a supported high-sitting position in bed or seated in an upright position in a chair when using PEP therapy.
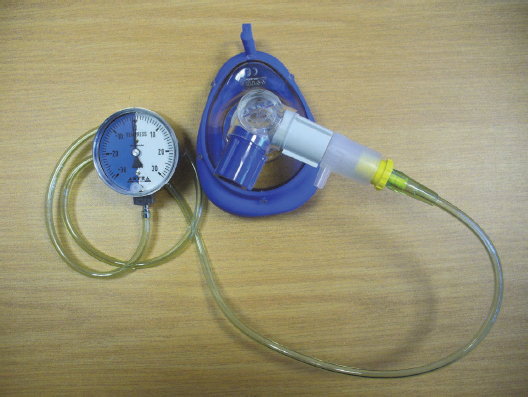
4.2.2.4.1.1. Continuous PEP therapy. Expiratory airway pressure remains at a pressure level above atmospheric pressure when continuous PEP therapy is applied. The PEP mask is a popular form of application of continuous PEP. The PEP mask consists of a one-way valve onto which expiratory (outflow) resistors of various diameters (ranges: 1 mm to 5 mm) can be attached. The opposite end of the resistor is attached via tubing to a manometer, through which the generated expiratory pressure is monitored (Figs 4.6A and B).
The PEP mask is a flow resistor device, as the expiratory pressure generated through the mask is determined by expiratory airflow and the diameter of the outflow resistor (Sehlin et al., 2007). The patient is instructed to seal the mask to the face and to perform a sequence of 10 breaths at a time. During each sequence of breaths the PEP mask keeps the alveoli pressurised, which adds benefit compared to methods in which positive pressure is only maintained for a few seconds during expiration (oscillatory PEP). The patient is instructed to perform two to three breathing sequences during one treatment session, with a pause period between each breathing sequence. The desired starting pressure to be generated through the mask is 10 cmH2O (Sehlin et al., 2007), but individual patient assessment is important to establish whether a patient is able to achieve this pressure level during the first session; lower starting pressures may be required, especially if the patient is in pain. Progression to higher levels of PEP should be done in subsequent treatment sessions to achieve the desired patient outcome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree