Physical Examination in Heart Failure
Kanu Chatterjee
The goals of physical examination in patients with suspected or established heart failure are as follows:
To determine the potential cause of heart failure
To assess the severity of heart failure
To determine the hemodynamic profile
To assess prognosis
During follow-up evaluation, to assess response to therapy
During clinical evaluation of a patient with suspected heart failure, it is desirable to establish a systematic approach every time a patient is examined. These various steps and sequences of physical examination are variable and depend on the examiner’s preference; however, if the same sequences are repeated every time the patient is examined, the chances of missing valuable information that can be obtained by physical examination can be minimized. I prefer the following steps:
General inspection
Examination of arterial pulse
Examination of venous pressure and pulse
Palpation of precordium and the epigastrum
Auscultation of heart sounds and murmurs
Examination of abdomen
Examination of the inferior extremities
Examination of the lungs
Recording of blood pressure and respiratory rate
Special examinations when indicated, such as fundo-scopic examination and the Valsalva maneuver
General Inspection
During evaluation of a patient with suspected or established heart failure, the presence or absence of respiratory distress, and types of altered respiration should be observed. Labored and uncomfortable breathing can be of cardiac or noncardiac origin. However, inability to lie down or associated dry, irritating cough with dyspnea in the supine position usually indicates pulmonary venous congestion. Typical Cheyne-Stokes respiration usually indicates severe heart failure. Although the mechanism of Cheyne-Stokes respiration is not entirely clear, it is associated with decreased chemoreceptor sensitivity. Although it occurs more frequently during sleep, Cheyne-Stokes respiration can occur when the patient is awake. Cheyne-Stokes respiration should be differentiated from Biot respiration. Bitot respiration is characterized by equal amplitude of inspiration during respiratory phase following apnea. Cheyne-Stokes respiration is characterized by gradually increasing amplitude of inspiration during the hyperpneic phase following phases of apnea. Sleep apnea may be obstructive or central, and both types of sleep apnea have been observed in patients with chronic heart failure. Biot respiration is usually associated with neurologic disorders, whereas Cheyne-Stokes respiration is more frequently observed in patients with heart failure.
During inspection, changes in skin color are also observed. A bluish discoloration of the skin usually indicates cyanosis. When cyanosis is observed, a distinction between central and peripheral cyanosis should be made.
Peripheral cyanosis is detected only in exposed skin, such as the lips, nose, earlobes, and extremities. Peripheral cyanosis usually indicates impaired peripheral perfusion due to low cardiac output or marked vasoconstriction. Central cyanosis is associated with a bluish discoloration of the tongue, uvula, and buccal mucous membrane and indicates either intrapulmonary or intracardiac right to left shunt resulting in increased proportion of desaturated hemoglobin greater than 3 g. The distribution of cyanosis can also provide a clue to the diagnosis of the underlying mechanism. If cyanosis along with clubbing is observed only in the inferior extremities and not in the upper extremities, Eisenmenger syndrome associated with patent ductus arteriosus should be suspected.
Peripheral cyanosis is detected only in exposed skin, such as the lips, nose, earlobes, and extremities. Peripheral cyanosis usually indicates impaired peripheral perfusion due to low cardiac output or marked vasoconstriction. Central cyanosis is associated with a bluish discoloration of the tongue, uvula, and buccal mucous membrane and indicates either intrapulmonary or intracardiac right to left shunt resulting in increased proportion of desaturated hemoglobin greater than 3 g. The distribution of cyanosis can also provide a clue to the diagnosis of the underlying mechanism. If cyanosis along with clubbing is observed only in the inferior extremities and not in the upper extremities, Eisenmenger syndrome associated with patent ductus arteriosus should be suspected.
Bronze- or slate-colored pigmentation of the skin suggests hemochromatosis, which may be associated with restrictive or dilated cardiomyopathy. Patients on chronic amiodarone therapy also develop similar discoloration of the skin, particularly following exposure to sunlight. This discoloration of the skin also may be seen in patients with carcinoid heart disease, another cause of heart failure. Acrosclerosis with thickened edematous and taut skin with or without sclerodactyly suggests systemic sclerosis, a syndrome that may be associated with pulmonary hypertension, pericarditis, right heart failure, systemic hypertension, restrictive cardiomyopathy, and dilated cardiomyopathy. Malar flush indicates the presence of severe pulmonary hypertension, regardless of the cause.
In patients with heart failure, nutritional status should be observed. The presence of cardiac cachexia or marked loss of skeletal muscle mass is usually evident from bitemporal wasting and indicates the presence of severe heart failure. However, hemodynamic abnormalities such as degree of reduction in cardiac output or in elevation of pulmonary venous pressure do not correlate with the presence or absence of cardiac cachexia. Cardiac cachexia in patients with heart failure is associated with a severe abnormality of neuroendocrine profile characterized by marked elevation of angiotensin II and cytokines such as tumor necrosis factor-α. Ascites and peripheral edema that also can be observed during inspection may indicate severe right heart failure or constrictive pericarditis; however, these findings also may be seen in other conditions such as cirrhosis of the liver and nephrotic syndrome.
The clinician should observe the patient for abnormal movement of the head and neck because such movement may yield information about the etiology or severity of heart failure. Lateral movements of the earlobes with each cardiac cycle strongly suggest severe tricuspid regurgitation. Bobbing of the head coincident with each heart beat is usually associated with severe aortic regurgitation. Finally, abnormalities of the eyes such as exophthalmus, lid-lag, stare, or periorbital edema may suggest the presence of thyroid abnormalities or another systemic disease.
Examination of the Arterial Pulse
During initial evaluation, all accessible arterial pulses should be examined. In the superior extremities, both brachial and radial and, if necessary, axillary pulses should be examined. In the inferior extremities, dorsalis pedis, posterior tibial, and femoral pulses should be examined bilaterally. In adult patients, an absent or diminished dorsalis pedis pulse may indicate atherosclerotic peripheral vascular disease that is associated with increased incidence of coronary artery disease. Loss of or decreased femoral pulse unilaterally or bilaterally most frequently indicates local obstructive lesions in adult patients. The radial femoral delay in young patients may indicate coarctation of the aorta. In adult patients, however, it most frequently suggests local obstructive peripheral vascular disease. Rarely in adult patients, severe pseudo-coarctation of the aorta may cause significant radial femoral delay.
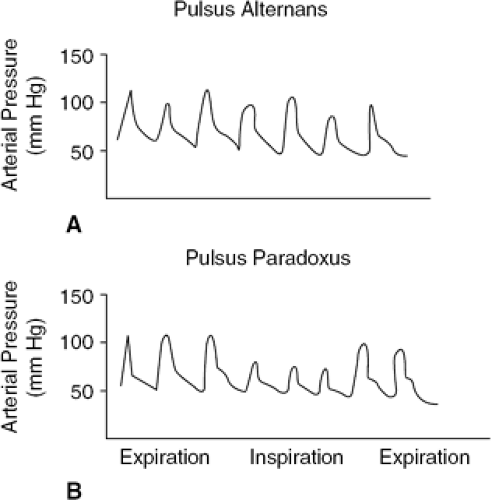
In a patient with suspected heart failure, peripheral arterial pulses are examined to detect any abnormalities of the character of the arterial pulse. Pulsus alternans is suspected when strong- and weak-amplitude pulses are appreciated with alternate beats in the presence of a regular pulse (Fig. 31-1). Pulsus alternans can be confirmed by measuring systolic blood pressure by sphygmometer. The phase I Korotkoff sound, which indicates peak systolic blood pressure, is higher with the stronger beats than with the weaker beats. The difference between the stronger and weaker beats represents the magnitude of the pulsus alternans. In some patients, the pulse with the weaker beat is not palpable, which is defined as total pulsus alternans. The precise mechanisms of pulsus alternans are not clear. However, for clinical purposes, pulsus alternans almost always indicates the presence of impaired left ventricular systolic function. Changes in contractile function, preload, and afterload contribute to persistence of pulsus alternans. Changes in left ventricular diastolic function due to abnormalities of ventricular filling also have been shown to contribute to the development and persistence of pulsus alternans. In the failing heart, however, changes in arterial pressure appear to
be the major determinant for the persistence of pulsus alternans. The failing heart is sensitive to altered afterload and resistance to left ventricular ejection. The increased arterial pressure associated with a strong beat increases the resistance to left ventricular ejection for the following beat, which is associated with decreased forward stroke volume and decreased arterial pressure, which reduces the resistance to left ventricular ejection for the following beat, allowing a larger stroke volume and increase in systolic blood pressure.
be the major determinant for the persistence of pulsus alternans. The failing heart is sensitive to altered afterload and resistance to left ventricular ejection. The increased arterial pressure associated with a strong beat increases the resistance to left ventricular ejection for the following beat, which is associated with decreased forward stroke volume and decreased arterial pressure, which reduces the resistance to left ventricular ejection for the following beat, allowing a larger stroke volume and increase in systolic blood pressure.
For clinical purposes, the presence of pulsus alternans indicates depressed left ventricular systolic function and thus allows the diagnosis of systolic ventricular failure. It should be realized, however, that the absence of pulsus alternans does not indicate the absence of impaired left ventricular systolic function. It is of interest to note that with improved left ventricular ejection fraction and increased stroke volume, pulsus alternans may resolve. In some patients with severe left ventricular failure with markedly reduced left ventricular stroke volume and relative hypotension, pulsus alternans may not be obvious. Furthermore, in some conditions such as hypertrophic cardiomyopathy or aortic valve stenosis, arterial pulsus alternans may not be apparent even when left ventricular pressure pulsus alternans is present. In clinical practice, however, if one can detect pulsus alternans it is likely that left ventricular ejection fraction is reduced and further evaluation should be undertaken to confirm the diagnosis of systolic left ventricular failure.
A substantial decrease in the amplitude of the arterial pulse during the inspiratory phase of respiration indicates the presence of pulsus paradoxus (Fig. 31-1). The pulsus paradoxus should be confirmed by measuring systolic blood pressure during the expiratory and inspiratory phases of respiration. A decrease in arterial pressure greater than 12 to 15 mm Hg during inspiration is regarded as pulsus paradoxus and is usually detectable by palpation of the peripheral pulses. The obvious pulsus parodoxus is an important physical finding for the diagnosis of cardiac tamponade. The inspiratory decrease in arterial pressure in tamponade results from the marked inspiratory decline of left ventricular stroke volume due to a decreased end-diastolic volume. During inspiration, there is an increase in venous return to the right atrium and the right ventricle. Due to increased intrapericardial pressure, the intraventricular septum shifts toward the left ventricle during inspiration (reversed Bernheim effect). This diastolic shift of the intraventricular septum toward the left ventricle decreases left ventricular preload, causing a reduction in its stroke volume and, therefore, systolic blood pressure. It should be appreciated, however, that pulsus paradoxus is frequently observed in patients with chronic obstructive pulmonary disease. Pulsus paradoxus is also occasionally observed in patients with pulmonary embolism, pregnancy, marked obesity, and partial obstruction of the superior vena cava. It is rarely encountered in patients with constrictive pericarditis. In hypertrophic obstructive cardiomyopathy, arterial pressure occasionally increases during inspiration (i.e., reversed pulsus paradoxus). The precise mechanism for this phenomenon is not clear.
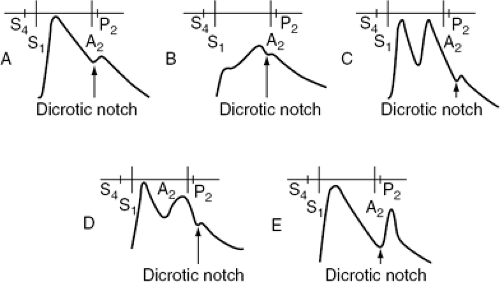
Analysis of the contour of the carotid pulse and peripheral arterial pulse can provide a useful indication regarding the etiology of left ventricular failure (Fig. 31-2). The delayed upstroke of the arterial pulse with anacrotic character and delayed peak is most frequently associated with hemodynamically significant aortic valve stenosis. It should be appreciated, however, that in elderly subjects, in whom vascular compliance is reduced, absence of the anacrotic pulse, decreased arterial pulse volume, or delayed peak of the carotid pulse does not necessarily indicate absence of significant aortic stenosis. The pulsus bisferiens character of the arterial pulse indicates predominant aortic regurgitation, although it can be appreciated in patients with obstructive hypertrophic cardiomyopathy. The pulsus bisferiens character is also observed in patients with mixed aortic stenosis and aortic regurgitation. However, when this finding is present, aortic regurgitation is usually the more dominant lesion. The dicrotic arterial pulse is difficult to differentiate at the bedside from pulsus bisferiens. The dicrotic pulse is appreciated in some patients with severe heart failure due to dilated cardiomyopathy and is usually associated with low cardiac output and increased systemic vascular resistance. On the other hand, a dicrotic pulse contour is also appreciated in patients with septic shock who have high cardiac output and low systemic vascular resistance. A dicrotic pulse can be present after aortic valve replacement and usually indicates depressed left ventricular ejection fraction postoperatively. However, in such patients echocardiographic evaluation is desirable to exclude aortic regurgitation due to the malfunction of the prosthetic valve causing pulsus bisferians, which can be misinterpreted as a dicrotic pulse.
During examination of the arterial pulse, it is desirable to evaluate the presence of arrhythmias, although the diagnosis of arrhythmias should always be confirmed by electrocardiography. The incidence of atrial fibrillation can be as high as 20% in patients with chronic congestive heart failure. Atrial fibrillation can be diagnosed at the bedside
by noting an irregular pulse. It should be emphasized, however, that atrial flutter or supraventricular tachycardia with variable atrioventricular block also may produce an irregular pulse. Differentiation between these arrhythmias needs to be made by electrocardiography. It is important to determine the heart rate and pulse rate in patients with atrial fibrillation to assess the magnitude of pulse deficit. It has been established that patients with systolic ventricular failure with reduced left ventricular ejection fraction may develop worsening heart failure due to rapid ventricular response, which may not be appreciated by examining the arterial pulse alone. The rapid ventricular response not only impairs left ventricular filling and produces worsening hemodynamics, but also can cause further depression of left ventricular contractile function (inverse force-frequency relationship). In a patient with atrial fibrillation with variable R-R cycle length, it is also desirable to assess the changes in the amplitude of the arterial pulse with changes in the preceding R-R intervals. If there is little or no change in the arterial pulse volume following a long R-R cycle length and a short R-R cycle length, diminished preload and contractile reserve should be suspected.
by noting an irregular pulse. It should be emphasized, however, that atrial flutter or supraventricular tachycardia with variable atrioventricular block also may produce an irregular pulse. Differentiation between these arrhythmias needs to be made by electrocardiography. It is important to determine the heart rate and pulse rate in patients with atrial fibrillation to assess the magnitude of pulse deficit. It has been established that patients with systolic ventricular failure with reduced left ventricular ejection fraction may develop worsening heart failure due to rapid ventricular response, which may not be appreciated by examining the arterial pulse alone. The rapid ventricular response not only impairs left ventricular filling and produces worsening hemodynamics, but also can cause further depression of left ventricular contractile function (inverse force-frequency relationship). In a patient with atrial fibrillation with variable R-R cycle length, it is also desirable to assess the changes in the amplitude of the arterial pulse with changes in the preceding R-R intervals. If there is little or no change in the arterial pulse volume following a long R-R cycle length and a short R-R cycle length, diminished preload and contractile reserve should be suspected.
In patients with established systolic ventricular failure not resulting from chronic left ventricular outflow tract obstruction or aortic regurgitation, the character of the arterial pulse is usually noninformative. The presence of pulsus alternans is the only finding that can suggest impaired left ventricular systolic and diastolic function. It should be also emphasized that absence of pulsus alternans does not exclude systolic ventricular failure, and it can be absent even in patients with severe left ventricular systolic dysfunction with hypotension and reduced stroke volume.
Examination of Central Venous Pressure and Pulse
The central venous pressure is usually examined with patients in a 45-degree semirecumbent position. However, when the venous pressure is low, it may be necessary to examine the patient lying supine or even in the Trendelenburg position. When the venous pressure is very high, it is sometimes necessary to examine the patient in the sitting position or in the standing position. It is preferable to examine both right and left internal jugular venous pulse and pressure. In elderly subjects, external jugular veins may be compressed by platisma muscle, which will cause spuriously high readings of venous pressure. In elderly patients, the left internal jugular venous pulse pressure can be higher than that of the right internal jugular vein because of the partial obstruction of the left innominate vein by an unfolded (ectatic) aorta. With inspiration and descent of the diaphragm, this partial obstruction may be relieved and pressures in both right and left internal jugular veins become equal.
The venous pressure is determined by adding 5 cm to the height of the venous column. It should be emphasized that to assess right atrial pressure, it is desirable to note first that there is a transmitted pulsation to the neck veins. In the era of cardiac transplantation when frequent biopsy is performed using the right internal jugular vein, partial right internal jugular venous obstruction can occur so that no pulsation of this vein is observed. At the bedside, venous pulsation is differentiated from carotid artery pulsation by inspection. The venous pulse is characterized by a sharp inward movement, whereas the arterial pulse is characterized by a sharp outward movement. When a patient is in sinus rhythm, inspection of the venous pulse demonstrates a double undulation character that is, however, lost in atrial fibrillation. The double positive waves that are observed in the venous pulse are A and V waves. In clinical practice, the inward movement or negative wave that is observed in the venous pulse is the y descent. It is difficult to recognize the x descent at the bedside. The venous pulsation also can be differentiated from the arterial pulsation with compression at the root of the neck, which obliterates the venous pulse but not the carotid pulse.
After recognizing the venous pulse and estimating the venous pressure, the character of the venous pulse is analyzed. If an unusually prominent A wave that precedes the carotid pulse upstroke is appreciated, conditions that increase resistance to right atrial emptying during atrial systole should be considered. Tricuspid valve obstruction is relatively uncommon in clinical practice. Right ventricular hypertrophy with or without right ventricular failure is a more important cause of a prominent A wave. In adult patients, a prominent A wave is frequently observed in the presence of a noncompliant right ventricle due to right ventricular hypertrophy, usually due to pulmonary hypertension. However, right ventricular hypertrophy due to right ventricular outflow tract obstruction may also increase resistance to right atrial emptying and, hence, a prominent A wave.
A prominent V wave usually indicates tricuspid regurgitation. However, in some patients with a noncompliant right atrium and increased right atrial venous return, the normal V wave may be accentuated even in the absence of tricuspid regurgitation. Severe tricuspid regurgitation is characterized not only by the presence of a prominent V wave, but also by a sharp y descent. Once tricuspid regurgitation is suspected, this diagnosis should be confirmed by auscultation, which reveals an early systolic or pansystolic regurgitant murmur along the lower left sternal border, which may radiate to the right side of the sternum or over the epigastrum. The murmur also increases in intensity during inspiration. Severe tricuspid regurgitation is frequently associated with systolic hepatic pulsation. After the diagnosis of tricupsid regurgitation has been confirmed, it is desirable to assess the intensity of the pulmonic component of the second heart sound to distinguish between primary and secondary tricuspid regurgitation. For clinical purposes secondary tricuspid regurgitation is defined if it is associated with pulmonary hypertension that can be diagnosed if the intensity of the pulmonic component of the second heart sound is increased.
When the venous pressure is elevated without a prominent x or y descent or only with obvious x descent, cardiac tamponade should be excluded. Cardiac tamponade is also associated with quiet precordium and occasionally distant heart sounds and no evidence of pulmonary hypertension. If the venous pulse demonstrates a sharp y descent along with elevated mean pressure, restrictive cardiomyopathy or
constrictive pericarditis should be considered. In both constrictive pericarditis and restrictive cardiomyopathy, the Kussmaul sign (a lack of decrease or increase in systemic venous pressure during inspiration) is observed. It needs to be appreciated that the Kussmaul sign also may be present in primary right ventricular failure such as right ventricular infarction, pulmonary embolism, and chronic tricuspid regurgitation. Restrictive cardiomyopathy can be associated with mitral and tricuspid valve regurgitation and pulmonary hypertension. The presence of tricuspid and mitral regurgitation with pulmonary hypertension favors the diagnosis of restrictive cardiomyopathy rather than constrictive pericarditis. However, further invasive and noninvasive investigations are necessary for the differentiated diagnosis of constrictive pericarditis and restrictive cardiomyopathy.
constrictive pericarditis should be considered. In both constrictive pericarditis and restrictive cardiomyopathy, the Kussmaul sign (a lack of decrease or increase in systemic venous pressure during inspiration) is observed. It needs to be appreciated that the Kussmaul sign also may be present in primary right ventricular failure such as right ventricular infarction, pulmonary embolism, and chronic tricuspid regurgitation. Restrictive cardiomyopathy can be associated with mitral and tricuspid valve regurgitation and pulmonary hypertension. The presence of tricuspid and mitral regurgitation with pulmonary hypertension favors the diagnosis of restrictive cardiomyopathy rather than constrictive pericarditis. However, further invasive and noninvasive investigations are necessary for the differentiated diagnosis of constrictive pericarditis and restrictive cardiomyopathy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree