A phase II dose-ranging study of the phosphodiesterase inhibitor K-134 in patients with peripheral artery disease and claudication
Brass EP, Cooper LT, Morgan RE, et al (Harbor-UCLA Ctr for Clinical Pharmacology, Torrance; Mayo Clinic, Rochester, MN; Kowa Res Inst, Morrisville, NC; et al) J Vasc Surg 55:381-389.e1, 2012§
J.D. Raffetto, MD
Evidence Ranking
A
Expert Rating
2
Abstract
Conclusions
K-134 was generally well tolerated. K-134 at a dose of 100 mg twice daily did not affect PWT according to the primary analysis, but K-134 and cilostazol both increased PWT when analyzed using a mixed-effects model and in the per-protocol population (Tables 1–4).
Table 1
Demographics of Study Patientsa
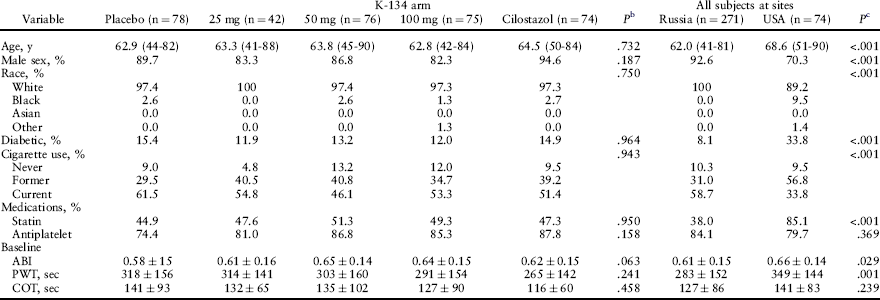
ABI, Ankle-brachial index; COT, claudication onset time; PWT, peak walking time.
aMean data are shown with the range or standard deviation. Data are for the modified intention-to-treat population, except for the 25-mg K-134 arm, for which data are from all randomized subjects. Data by country includes the modified intention-to-treat population and the 42 subjects randomized to 25 mg of K-134. P values for continuous variables were calculated from a one-way analysis of variance, and the χ2 test was used for categoric variables.
bFor differences between treatment arms.
cFor differences between countries.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


