Treatment
Recommended
Strength of recommendation
Quality of evidence
Pharmacologic therapies
Corticosteroid monotherapy
No
Strong
⊕○○○
Colchicine
No
Strong
⊕○○○
Cyclosporine A
No
Strong
⊕○○○
Corticosteroid + immunomodulatory
No
Strong
⊕○○○
Corticosteroid + azathioprine + acetylcysteine
Majority – no
Weak
⊕⊕○○
Minority – may be a reasonable choice
2015 Guidelines
No
Strong
Taken together, there was no evidence showing the usefulness of the combination of corticosteroid and immunosuppressant for the treatment of IPF. Considering the possibility that this therapy may induce acute exacerbation associated with dose reduction and side effects such as a complicated infection which occurs with long-term usage, the treatment with corticosteroid and immunosuppressant is not recommended in definite IPF patients.
10.3 Therapy for IPF in Our Clinical Practice
A study that aimed at elucidating the actual medical practice concerning IPF in Japan entitled “A prospective investigation research for diffuse pulmonary disease, Project on Measures for Intractable Diseases, Health and Labour Science Research Grant” was registered on the Internet, and a prospective epidemiological study was conducted [12]. Information obtained from a multicenter study including the therapeutic regimen, clinical findings, and the clinical course of patients with IIPs including IPF was actively entered in a database on the Internet. As a result, 321 IPF patients from 19 medical facilities were registered. Regarding the therapeutic regimen for IPF patients and a change in the regimen (see Fig. 10.1), the majority of IPF patients were untreated (78.7 %) by the end of fiscal year 2008 when pirfenidone was approved, but the untreated rate among IPF patients decreased by 44.6 % between 2009 and the end of fiscal year 2013. Pirfenidone has been used as a therapy for IPF patients (32.9 % between 2009 and the end of fiscal year 2013) including pirfenidone monotherapy (17.4 %), and therefore this medication plays a key role in the treatment of IPF in Japan. On the other hand, the use of corticosteroid monotherapy for IPF patients showed a slight increase from 6.2 to 7.5 %; likewise, the use of the combination of corticosteroid and immunosuppressant slightly increased from 11.2 to 13.1 %. These results show that the combination of a corticosteroid and immunosuppressant is used conditionally as symptomatic therapy for IPF in our medical practice with awareness of the side effects of each medication.
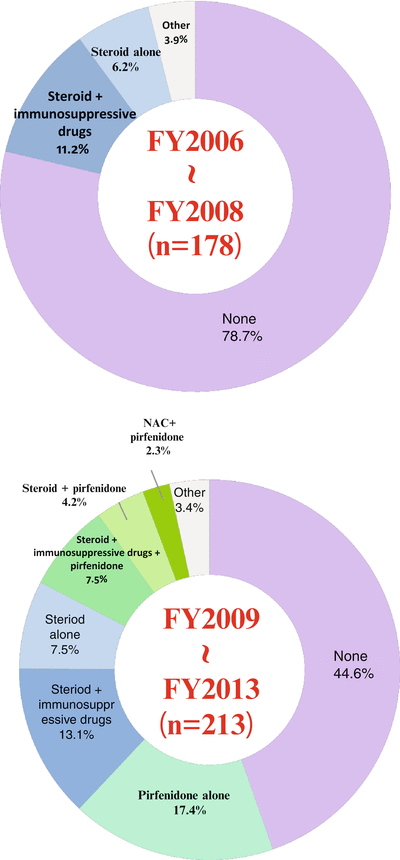

Fig. 10.1
The treatments and their changes in IPF
10.4 Pharmacological Effects and Side Effects from the Use of Corticosteroid and Immunosuppressant
10.4.1 Corticosteroids
The anti-inflammatory effects of corticosteroids are well known for their genomic mechanism producing biological actions. After the corticosteroids form complexes with glucocorticoid receptors (GCRs) inside the cytoplasm, the complexes translocate to the nucleus and bind to glucocorticoid-response elements on DNA. Once GCRs that had translocated into the nucleus bind to negative glucocorticoid-response element, the mRNA transcription of various cytokines involved in inflammation is inhibited. On the other hand, when GCRs translocate into the nucleus and bind to positive glucocorticoid-response element on DNA, the mRNA transcription of anti-inflammatory proteins is upregulated. The binding of transcription factors including nuclear factor-κB and AP-1 to DNA is inhibited, resulting in interference of cytokine production [16]. The amount of corticosteroid that would saturate corticosteroid receptors in an adult human is approximately 60 mg of prednisolone, although there are individual differences. In contrast, high-dose corticosteroid therapy is thought to act through a non-corticosteroid receptor-mediated mechanism, so-called non-genomic mechanism [17], which is entirely different from the genomic mechanism and has an onset of effect between a few seconds and a few minutes. Although the details are unknown at this time, there are two kinds of non-genomic mechanisms: nonspecific effects that directly act on cell membrane fluidity and specific effects that act on a specific receptor. Corticosteroid pulse therapy can be expected to have stronger genomic and non-genomic effects and have an impact on inflammatory cells, alveolar epithelial cells, T lymphocytes, vascular endothelial cells, etc. [18, 19]. Meanwhile, because corticosteroids do not inhibit the production of basic fibroblast growth factor and transforming growth factor-β (TGF-β) in bleomycin-induced murine pulmonary fibrosis [20], they have no antifibrotic actions. The following side effects of corticosteroids are important: diseases induced by infectious diseases (particularly tuberculosis, fungus, cytomegalovirus, Pneumocystis pneumonia, etc.), peptic ulcer, diabetes, mental deterioration, hypertension, secondary adrenocortical insufficiency, osteoporosis, aseptic necrosis of the femur and others, myopathy, glaucoma, cataract, thrombosis, endocrine abnormality, and so on. Because the above major side effects influence disease prognosis, whether the therapeutic effect is beneficial to the patient or not needs to be carefully pondered when such a side effect occurs. In addition, a decision must be made as to whether patients will continue the therapy with corticosteroid, reduce the dose, or discontinue this therapy. When a corticosteroid is administered for a long period of time, the combined treatment of sulfamethoxazole and trimethoprim is necessary to prevent Pneumocystis pneumonia. Because postmenopausal women and elderly people are vulnerable to the development of osteoporosis and compressed fracture, a medication such as bisphosphonate is also required. In contrast, minor side effects of corticosteroid administration include hirsutism, acne, moon-shaped face, extravasation of blood into the skin, purpura, and so on, but these side effects are sometimes not severe, and the physician may not recommend reducing the dose or discontinuing the drug.
10.4.2 Immunosuppressants
Generally, an immunosuppressant is used for the treatment of various interstitial pneumonias other than IPF in the following cases: patients who have no response to corticosteroids, those who experienced severe side effects of corticosteroid, and those who are at high risk of developing side effects with corticosteroid. In the United States and Europe, cyclophosphamide and azathioprine are often used for treatment, but cyclosporine A is also used in Japan.
10.4.2.1 Cyclophosphamide
Cyclophosphamide is classified as an alkylating compound that is activated by hepatic microsomal enzymes and exhibits pharmacological action. The inhibitory effects of cyclophosphamide on DNA synthesis act on the cell cycle in a nonspecific manner. Cyclophosphamide shows a stronger inhibitory action against B lymphocytes than T lymphocytes. The general dosage required for cyclophosphamide is 1.0–2.0 mg/kg/day (ideal body weight, highest dose: 150 mg/day). The medication is initiated at 50 mg/day and increased by 25 mg every 7–14 days as needed. Because the onset of the therapeutic effect is usually more than 3 months after starting this medication, the medication needs to be continued for at least 6 months or longer as long as there are no severe side effects. Some side effects of cyclophosphamide are bone marrow suppression, hemorrhagic cystitis, second primary cancer, hair loss, a feeling of sickness, stomatitis, diarrhea, and hepatic impairment associated with cholestasis. Pulmonary fibrosis has also been reported although it is rare. The medication is suspended or the dose is reduced by half when the white blood cell count falls below 4,000/mm3 or platelet count falls below 100,000/mm3. Patients drink adequate fluids to prevent hemorrhagic cystitis, establish urine flow, and take a urine test monthly. When hemorrhagic cystitis occurs, the medication is discontinued.
10.4.2.2 Azathioprine
Azathioprine, which is classified as an antimetabolic drug, is transformed into 6-mercaptopurine in the liver and is later physiologically activated. Azathioprine is a medication that acts specifically on the cell cycle and inhibits purine synthesis by acting on the DNA synthetic phase. Its immunosuppressive effect is mainly caused by suppression of the proliferation of T lymphocytes. The general dosage required for azathioprine is 2.0–3.0 mg/kg/day (ideal body weight, highest dose: 150 mg/day). The medication is initiated at 50 mg/day and the dose is increased by 25 mg every 7–14 days as needed. Side effects are bone marrow suppression, a feeling of sickness, vomiting, gastrointestinal symptoms such as diarrhea, and hepatic impairment. The medication is suspended or its dose is reduced by half when the white blood cell count falls below 4,000/mm3 and the platelet count falls below 100,000/mm3. Patients undergo a hepatic function test monthly, and the medication is later suspended, or its dose is reduced when the measured value of AST and ALT reaches more than three times the upper limit of a normal hepatic function.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


