Carotid Artery
In the United States, about 750,000 strokes occur each year, and 88% of these are due to ischemia (
5). Stroke is the third leading cause of death in Western society, and one-third of stroke patients remain institutionalized at 3 months after stroke. One-third of the cerebral ischemic events are associated with a significant obstructive disease in the extracranial carotid artery—a nidus for plaque formation and rupture that may cause recurrent symptoms of cerebroembolism.
Since the publication of the North American Symptomatic Carotid Endarterectomy Trial (NASCET) (
6), European Carotid Surgery Trial (
7), and the Endarterectomy for Asymptomatic Carotid Atherosclerosis Study (ACAS) (
8), carotid artery endarterectomy (CEA) has been established as the gold standard for the revascularization of symptomatic and asymptomatic carotid artery disease. However, the patients enrolled in these clinical trials were highly selected, and the trial results did not reflect real-world practice (
9,
10). Many patients who require carotid revascularization are
considered high-risk for surgical revascularization and would have been excluded from the original trials. Indeed, by analyzing the Medicare data from the years when these trials were performed (1992-1993), those treated in the trial hospitals outside of the research protocol and those treated in non-trial hospitals had much higher mortality rates than those treated within the clinical trial protocol (
9).
Percutaneous carotid artery revascularization provides a less invasive alternative to CEA. Endovascular treatment obviates the need for general anesthesia, eliminates the risk of cranial nerve injury or neck hematoma, and allows quicker recovery when compared with CEA. In the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) (
11), the clinical outcomes of endovascular revascularization (76% angioplasty alone, 26% with adjunctive stent placement) were similar to those of CEA, whereas the incidences of cranial nerve injury (0% versus 9%) and hematoma requiring surgical exploration (1% versus 7%) were significantly higher in the surgical group. The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) study randomized patients with high surgical risk (
Table 20.1) to undergo either CEA or carotid stenting using PRECISE (Cordis, Miami) with an adjunctive Angioguard (Cordis, Miami) emboli protection device (EPD) (
12). In this study, carotid stenting was associated with a significantly less composite endpoint of death, nonfatal stroke, and myocardial infarction (MI) at 30 days (5.8% versus 12.6%,
p = 0.047) and at 1 year (11.9% versus 19.9%,
p = 0.048) when compared with CEA (
12,
13). The analysis of the SAPPHIRE stent registry, which included those patients turned down by surgeons, suggested that the 1-year outcomes of these patients treated with carotid stents were similar to those who were randomized in the study (7.8% at 30 days and 15.8% at 1 year). Subsequent to the SAPPHIRE study, a number of high-surgical risk registries have been established involving various combinations of self-expanding stents and EPDs, and they concluded similar outcomes as those reported in the SAPPHIRE study. While ongoing clinical trials comparing the two strategies are still taking place (e.g., CAVATAS II, CARESS, SPACE, etc.), carotid stenting with EPD should be adopted as the treatment of choice for carotid artery revascularization for symptomatic and asymptomatic surgical high-risk patients.
The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) is a randomized clinical trial funded by the National Institutes of Health and the National Institute of Neurological Disorders and Stroke. CREST is designed to compare carotid stenting with CEA among symptomatic surgical low-risk patients (
14). The trial is currently in progress and is anticipated to be completed within the next 5 years at the time of this writing.
Importance of Emboli Protection Device
Distal embolization has been the Achilles’ heel of carotid stenting. The dislodgement of atherosclerotic debris during
plaque rupture by balloon, platelet activation, thrombus formation, and spasm of distal arterioles may lead to stroke during carotid intervention (
15). Using transcranial Doppler technique during percutaneous carotid intervention, microembolism is detectable throughout the procedure, beginning from catheter placement, but balloon dilatation and stent deployment are responsible for most of the signals detected (
16,
17). Over the past several years, a number of EPDs have become available in clinical practice or within the context of clinical investigations (
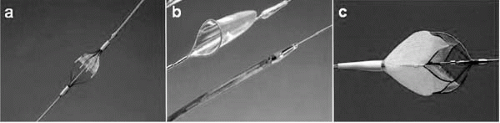
Fig. 20.2). Current EPDs can be classified according to three fundamental designs:
Flow-through filter (Angioguard [Cordis, Warren, New Jersey], Accunet [Guidant, Santa Clara, California], FilterWire EX [Boston Scientific, Santa Clara, California], and NeuroShield [MedNova, Redwood City, California])
Balloon occlusion GuardWire (Medtronic, Minneapolis)
Flow reversal with proximal balloon occlusion (Parodi Anti-Embolism System [ArteriA Medical Science Inc., San Francisco])
The efficacies of using distal balloon occlusion and filters in preventing distal embolization have been proven in the percutaneous revascularization of saphenous vein grafts (
18,
19). Thus, by extrapolation of these data, it is assumed that EPDs enhance the safety of carotid stent procedures, and they have become a standard adjunctive device during carotid stenting in our clinical practice (
Table 20.2).
By its design, each of the EPDs has logistical issues. The GuardWire is associated with prolonged cerebral ischemia during balloon occlusion, and the patient may not tolerate the procedure, especially in the presence of contralateral carotid occlusion. In addition, angiographic recording is not possible during intervention with this device. Furthermore, the “suction shadow,” occurring underneath the occlusive balloon and inaccessible to the aspiration catheter, may allow embolization of the trapped particles to the distal arterial bed upon balloon deflation. The pore size of current filters varies from 80 to 100 μm (Angioguard) to 120 μm (NeuroShield). The size of the atherosclerotic debris collected ranges from 120 to 3,000 μm (medium 580 μm) (
20,
21). Although particles <100 mm in size may pass through a filter, their clinical importance is unknown. When a filter is overwhelmed by large particles, flow in the carotid artery slows and filtration ceases. As with occlusion balloons, aspiration must be performed to remove the stagnant column of blood and debris before retrieving the filter.
Interventional Techniques
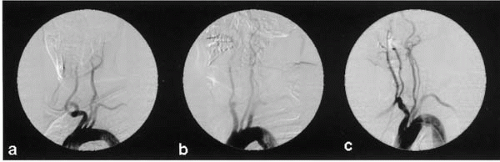
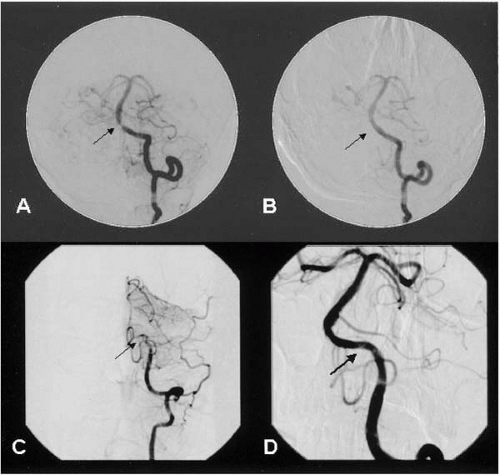
Diagnostic cerebral angiography includes aortic arch angiography and four-vessel selective angiography using digital subtraction technique. The aortic arch angiogram provides important information, including the location of the origins of the great vessels, unexpected anatomic variants, and previously unidentified proximal lesions in the great vessels (
Fig. 20.3). A 4 Fr Bernstein catheter, a 5 Fr right Judkins, Vitek, or Simmons catheter (Cordis, Miami), together with a 190 cm 0.035-inch stiff-angled glidewire is recommended to perform selective angiography. Meticulous technique is needed during diagnostic cerebral angiography, because it is associated with a significant (up to 1%) stroke risk (
22).
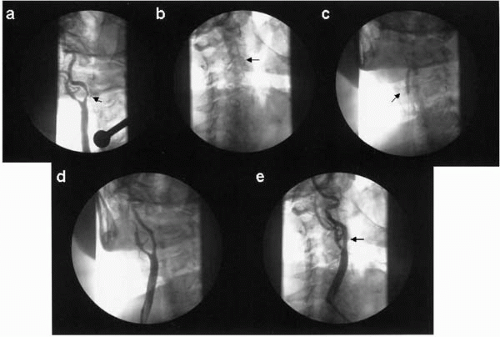
To perform an interventional procedure in an extracranial carotid artery, the patient should be well hydrated and antihypertensive medications should be held on the day of the procedure (
Fig. 20.4). Aspirin 81 to 325 mg and clopidogrel 300 mg should be given at least 24 hours before the procedure. A 6 Fr Shuttle sheath (Cook, Bloomington, Indiana) is typically exchanged for the diagnostic catheter over a 300 cm stiff Amplatz wire. Alternatively, a 5 Fr diagnostic catheter can be placed within a Shuttle sheath or an 8 Fr guide catheter (e.g., Headhunter-1) and, in a coaxial manner, this catheter acts as an introducer for the guide catheter to advance atraumatically over a glidewire into the arterial segment proximal to the target lesion. The diagnostic catheter and the glidewire then can be removed.
Anticoagulation using unfractionated heparin (activated clotting time 250 to 300 seconds) or bivalirudin is then provided. An EPD (e.g., FilterWire, Angioguard, GuardWire) then is advanced across the lesion and should be deployed within the straight segment of the precavernous portion of the distal extracranial internal carotid artery. Balloon predilatation can be performed using a 4.0 mm balloon catheter. If an EPD cannot cross a lesion initially, a 0.014-inch floppy guidewire can be used, and the lesion can be inflated with a small (2.0 mm) coronary balloon catheter before a repeat attempt to advance the EPD across the lesion. Atropine 0.5 to 1.0 mg is administered prophylactically to minimize the incidence of transient severe bradycardia during stretching of the carotid body if the resting heart rate is <60 beats/min. Using the guidance of bony landmarks or an angiographic roadmap, a self-expanding stent then is positioned and carefully deployed across the lesion. Postdilatation using a balloon catheter that matches the reference diameter of the internal carotid artery then is performed. For a carotid bifurcation lesion, the diameter of the self-expanding stent should match the diameter of the common carotid artery, and the length of the stent should be chosen to fully cover the lesion. The EPD then is retrieved, and angiography is performed for the target lesion as well as the intracerebral arteries supplied by the vessel in order to detect any distal embolization. When slow flow is noted on the angiogram before filter retrieval, this usually represents the filter being overwhelmed by the embolized material, or fibrin; a 125 cm-long diagnostic catheter or an aspiration catheter can be advanced over the EPD to aspirate the stagnant blood column before retrieving the filter. Access closure is usually recommended. The operator should then perform a brief neurologic examination before transferring the patient to the recovery area.
Postprocedure Care
Carotid body stimulation results in transient bradycardia and sustained hypotension. Aggressive hydration with normal saline should be provided; oral supplement (pseudoephedrine) or Neo-Synephrine can be provided to maintain the target blood pressure. Blood pressure is typically normalized within 12 to 24 hours, but occasionally it may take longer. Hyperperfusion syndrome occurs as a result of chronic maximal intracerebral vasodilatation and blunting of the autoregulatory response, and this occurs more frequently in the presence of a contralateral carotid artery occlusion. The patient may complain of headache in the presence of high systolic blood pressure (>140 mm Hg), in the absence of focal neurologic deficit. Therefore, intravenous nitroglycerin or nitroprusside should be titrated to maintain the blood pressure between 90 to 120 mm Hg. Within 24 hours of the procedure, independent neurologic assessment should be carried out by a stroke neurologist, and baseline carotid ultrasound and Doppler should be performed. Typically, a patient can be discharged within 24 hours postprocedure on lifelong aspirin and 30 days to 6 months of clopidogrel. Scheduled follow-up at 1, 6, and 12 months includes carotid ultrasound at 6- to12-month intervals.
Restenosis after carotid artery stenting has been reported in 2% to 3% of all patients in a large series (
4). The treatment of carotid in-stent restenosis includes repeat balloon angioplasty (BA) or stenting, although their efficacies have not been studied. Brachytherapy recently has been reported as a safe and feasible therapeutic option, and its effectiveness require a more systematic assessment in larger series (
23).