14 Peripheral Vascular Intervention
• Age less than 50 years, with diabetes and one other atherosclerosis risk factor (smoking, dyslipidemia, hypertension, or hyperhomocysteinemia)
• Age 50 to 69 years and a history of smoking or diabetes
• Symptoms with exertion involving the lower extremities (suggestive of claudication) or ischemic rest pain
• Abnormal lower extremity pulse examination
• Known atherosclerotic coronary, carotid, or renal artery disease
Patient Selection for Peripheral Endovascular Intervention
• Any exertional limitation of the lower extremity muscles or any history of impaired ambulation. This limitation may be described as cramping, fatigue, aching, numbness, or pain. The primary site(s) of discomfort in the buttock, thigh, calf, or foot should be documented, along with the relation of such discomfort to rest or exertion.
• Any poorly healing or nonhealing wounds of the legs or feet.
• Any pain at rest localized to the lower leg or foot and its association with the upright or recumbent positions.
• Postprandial abdominal pain that is provoked by eating and associated with weight loss.
• Family history of a first-degree relative with an abdominal aortic aneurysm.
The patient history should include questions that try to distinguish PAD from diseases that have a non-PAD etiology (Table 14-1).
Table 14-1 Questions Used to Distinguish PAD Symptoms From Non-PAD
| Pain and location | Do you have pain or discomfort? Where is the discomfort? |
| Consistency | Is the pain the same every time? |
| Severity | How much can you do before the pain starts? Have your activities changed because of the pain? |
| Character and quality | What does the discomfort feel like? Can you describe it? |
| Exacerbation | What makes the pain worse? What relieves the pain? |
PAD, peripheral arterial disease.
Intermittent claudication is the most classic manifestation of PAD. In 1962 epidemiologist Geoffrey Rose designed a questionnaire to assess the incidence and prevalence of intermittent claudication in large epidemiologic studies. The sensitivity of the Rose claudication questionnaire approaches approximately 10% to 30%; however, similar questions (and modifications to the Rose questionnaire) are still very helpful in diagnosing intermittent claudication (Table 14-2).
Table 14-2 Rose Questionnaire: Symptoms of Classic Intermittent Claudication
| Calf pain caused by exertion that: |
True vascular claudication must be distinguished from “pseudoclaudication” caused by severe venous obstructive disease, chronic compartment syndrome, lumbar disease and spinal stenosis, osteoarthritis, and inflammatory muscle diseases. The characteristic features of pseudoclaudication that distinguish it from claudication are summarized in Table 14-3.
Table 14-3 Distinguishing Characteristics Between Claudication and Pseudoclaudication
| Claudication | Pseudoclaudication | |
|---|---|---|
| Characteristic of discomfort | Cramping, tightness, aching, fatigue | Same as claudication plus tingling, burning, numbness |
| Location of discomfort | Buttock, hip, thigh, calf, foot | Same as claudication |
| Induced by exercise | Yes | Variable |
| Reproducible with distance walked | Consistent | Variable |
| Occurs with standing | No | Yes |
| Actions that provide relief | Standing | Sitting, changing position |
| Time until relief | <5 min | <30 min |
Symptoms of intermittent claudication classically start distally within a muscle group (below the stenosis) and then ascend with continued activity. Rest pain that occurs with leg elevation and is relieved paradoxically by walking may suggest severe PAD (as the effects of gravity increase arterial perfusion of muscle groups). Critical PAD may present as tissue ulceration and gangrene. The ACC/AHA guidelines suggest that individuals with PAD present in clinical practice with distinct syndromes (Table 14-4).
Table 14-4 Distinct syndromes of PAD
| Individuals with peripheral arterial disease (PAD) present in clinical practice with a variety of distinct syndromes: |
| Asymptomatic: Without obvious symptomatic complaint (but usually with a functional impairment) |
| Classic claudication: Lower extremity symptoms confined to the muscles with a consistent (reproducible) onset with exercise and relief with rest |
| “Atypical” leg pain: Lower extremity discomfort that is exertional but that does not consistently resolve with rest, consistently limit exercise at a reproducible distance, or meet all Rose questionnaire criteria |
| Critical limb ischemia: Ischemic rest pain, nonhealing wound, or gangrene |
| Acute limb ischemia: The five “P”s, defined by the clinical symptoms and signs that suggest potential limb jeopardy: pain, pulselessness, pallor, paresthesias, paralysis (and polar, as a sixth “P”) |
The physical exam defines the location, severity, and etiology of PAD and its symptoms. Arterial pulse intensity should be assessed and should be recorded numerically, as shown in Table 14-5. Table 14-6 outlines some of the important findings on the physical exam of the legs.
Table 14-5 Gradation of Arterial Pulse
| Numerical Gradation | Clinical Assessment |
|---|---|
| 0 | Absent |
| 1 | Diminished |
| 2 | Normal |
| 3 | Bounding |
Table 14-6 Physical Exam Findings of Peripheral Arterial Disease
Peripheral Vascular or Endovascular Intervention
1. Performed using local anesthesia, enabling the treatment of patients who are at high risk for general anesthesia.
2. Lower morbidity and mortality compared to surgical revascularization.
3. Earlier ambulation on the day of treatment and earlier return to normal activity within 24 to 48 hours.
4. Endovascular therapies may be repeated if necessary, generally without increased difficulty or increased patient risk compared to the first procedure.
5. Prior angioplasty does not preclude surgery if required at a later date.
Vascular Access
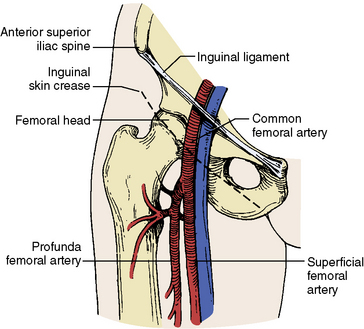
Successful endovascular intervention requires appropriate choice of vascular access. In most cases, access is obtained using a 21-gauge needle and 0.18-inch wire (4F micropuncture set). The retrograde approach to the common femoral artery (CFA) is the most frequently used vascular access. The inguinal crease is highly variable in relation to the CFA bifurcation in up to 75% of patients. Identifying the femoral head under fluoroscopy is very helpful (Fig. 14-1), as this will help ensure puncture of the vessel above the CFA bifurcation and below the inguinal ligament. Vascular access with the use of ultrasound imaging is also safe and effective as this allows direct imaging of the vessel.
The majority of PVIs can be performed from several access sites (Table 14-7). On most occasions the location of the lesion (to be intervened upon) will determine the most appropriate access site.
Table 14-7 Arterial Access for Different Vascular Territories
| Vascular Access | Artery(ies) to Revascularize |
|---|---|
| Retrograde CFA | Arch vessels, renal, & mesenteric |
| Contralateral CFA | Contralateral iliacs, CFA, PFA, SFA, popliteal |
| Antegrade CFA | Mid-distal femoral, popliteal, infrapopliteal |
| Brachial/radial artery | Renal (caudal takeoff), mesenteric, iliac arteries |
| Retrograde popliteal artery | SFA and iliac artery |
CFA, common femoral artery; PFA, profunda femoris artery; SFA, superficial femoral artery.
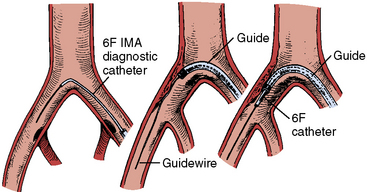
Retrograde CFA access permits selective angiography and intervention of the contralateral pelvic and lower extremity vessels. After gaining retrograde access (Fig. 14-2) to the CFA, the contralateral iliofemoral system is reached by placing a diagnostic catheter with an acute bend at the tip (usually an internal mammary artery [IMA] or Simmons catheter) at the aortic bifurcation. The catheter is manipulated so that the tip “engages” the ostium of the contralateral common iliac artery. A stiff-angled 0.035-inch Glidewire (Terumo Medical Corporation, Somerset, NJ) is then carefully steered to the femoral artery and the diagnostic catheter is advanced over the Glidewire into the CFA. The Glidewire is then exchanged through the diagnostic catheter for a stiff guidewire (Amplatz extra-stiff, Cook, Bloomington, IN), which is advanced into the distal femoral artery. The diagnostic catheter is then removed, leaving the extra-stiff wire in place. A crossover sheath (6 F–8 F) may then be advanced over the stiff guidewire and positioned in the contralateral CFA. This allows contrast injection during lesion dilation and backup support for crossing lesions. This type of sheath manipulation may be very difficult with individuals who have an acute angle between the origin of the common iliac arteries or those who have aortofemoral bypass grafts.
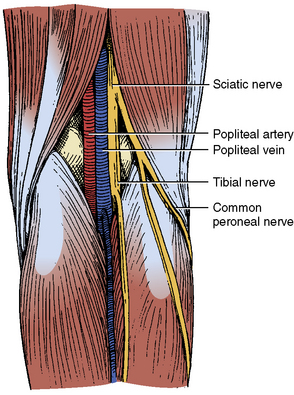
Retrograde popliteal artery access can be useful when trying to cross an occluded SFA. It is important to be aware of the anatomical relationship between the popliteal artery and vein. At the level of the joint space, the artery courses anterior to the vein, whereas, at approximately 6 cm cephalad to the joint space, the artery is medial to the vein (Fig. 14-3). For popliteal arterial puncture, the vessel should be free of significant disease and larger than 4 mm in diameter. Prior angiography and/or color-flow duplex imaging may provide useful information regarding puncture of this vessel. The first step is to gain contralateral CFA access in order to provide contrast injections to help visualize the target popliteal artery. The CFA sheath is secured in place and the patient is turned to the prone position. Contrast injections performed through the contralateral CFA sheath allow fluoroscopic visualization of the popliteal artery. In this way a micropuncture needle is directed obliquely from medial to lateral so that the artery is entered approximately 5 to 6 cm above the joint space. A 0.035-inch floppy guidewire is then advanced into the popliteal artery, and a 4F to 6F sheath is then inserted.
Nonselective Abdominal Angiography
Nonselective angiography requires knowledge of image angulations and vascular anatomy to better define disease involving the aortovisceral vessel junction, and other ostial disease. Table 14-8 depicts some useful angiographic views for different vascular territories.
Table 14-8 Most Useful Angiographic Views for Different Vascular Territories
| Artery or Vascular Territory | Angiographic View |
|---|---|
| Aortic arch | 30–60 degrees LAO (with slight cranial angulation) |
| Brachiocephalic vessels (origin) | 30–60 degrees LAO |
| Subclavian | AP, ipsilateral oblique with caudal angulation |
| Vertebral origin | AP, ipsilateral oblique with cranial angulation |
| Carotid extracranial | Lateral, AP, ipsilateral oblique |
| Renal arteries (origin) | AP, 5–30 degrees ipsilateral oblique |
| Mesenteric arteries (origin) | Lateral or steep RAO |
| Iliac artery | Contralateral 20–45 degrees oblique and 20o caudal |
| CFA, SFA, and PFA arteries | Ipsilateral 30–60 degrees oblique |
| Femoropopliteal | AP |
| Infrapopliteal trifurcation and runoff | AP |
AP, anteroposterior; CFA, common femoral artery; LAO, left anterior oblique; PFA, profunda femoris artery; RAO, right anterior oblique; SFA, superficial femoral artery.
Aortoiliac Intervention
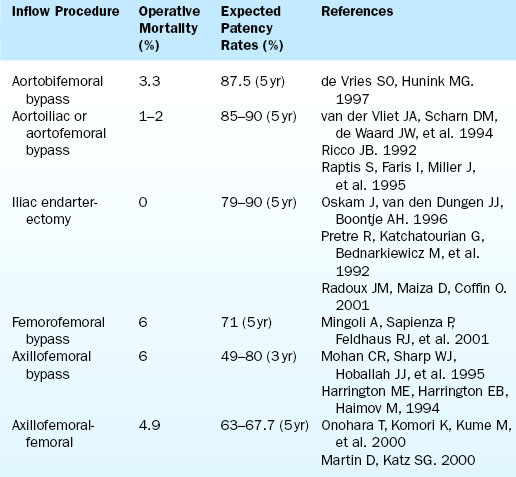
There are various types of aortoiliac occlusive disease and procedures to surgically treat them. The patency rate for these procedures is documented in Table 14-9. Most commonly, the lesions of greatest hemodynamic consequence are located in the iliac arteries. The most effective surgical procedure for the treatment for this type of atherosclerotic disease, and the resultant buttock and thigh claudication, is aortobifemoral bypass. If the aortoiliac lesions are confined to the area of the aortic bifurcation, localized aortoiliac endarterectomy may be considered. Less invasive approaches may be appropriate for patients with adequate aortic flow but stenosis or occlusion of both iliac vessels. Such patients may not be candidates for aortobifemoral bypass because of comorbid cardiovascular disease. If endovascular treatment of one iliac artery is possible and can achieve good results, then a subsequent endarterectomy, unilateral iliofemoral bypass, or femoral-femoral bypass can be considered. In the absence of an inflow stenosis within the iliac arteries, this procedure can provide flow to both lower extremities and eliminate the symptoms of claudication.
Endovascular reconstruction options include the following:
• Percutaneous transluminal angioplasty (PTA)
• Directional atherectomy, laser atherectomy, cutting balloon angioplasty, cryoplasty
• Avoiding complications of general anesthesia
• Avoiding surgical incision and subsequent wound healing complications
• Early recovery and ambulation
Iliac Artery Intervention
Indications
• A predicted or observed lack of adequate response to exercise therapy and claudication pharmacotherapy
• Presence of a severe disability, either being unable to perform normal work or having very serious impairment of other activities important to the patient
• Absence of other disease that would limit exercise even if the claudication was improved (e.g., angina or chronic respiratory disease)
• The individual’s anticipated natural history and prognosis
• The morphology of the lesion (must be such that the appropriate intervention would have low risk and a high probability of initial and long-term success)
• Provisional stent placement is indicated for use in iliac arteries as salvage therapy for suboptimal or failed result from balloon dilation (e.g., persistent gradient, residual diameter stenosis >50%, or flow-limiting dissection).
• Stenting is effective as primary therapy for common iliac artery stenosis and occlusions.
• Stenting is effective as primary therapy in external iliac artery stenosis and occlusions.
Technique
Balloon-expandable stents are preferred when a precise stent placement is required (ostial lesions), and self-expanding stents are preferred when precision is not a critical factor and the vessel tapers in size (Fig. 14-4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree