Peripheral Artery Disease
Lower extremity peripheral artery disease (PAD) is a marker of systemic atherosclerosis and has been estimated to affect approximately 8 to 12 million Americans.1 PAD is associated with functional and quality of life (QOL) impairment, increased risk of progressive limb ischemia, and increased risk of cardiovascular ischemic events (i.e., myocardial infarction [MI] and stroke) and mortality. Although screening for PAD is simple, easy, and inexpensive using the ankle–brachial index (ABI), PAD remains an underdiagnosed and undertreated health condition. Treatment of the PAD patient requires attention to leg symptoms and functional capacity as well as aggressive cardiovascular risk reduction therapies.
DEFINITION
Atherosclerotic peripheral vascular disease includes a diverse group of disorders that lead to progressive stenosis, occlusion, or aneurysmal dilation of the aorta and its noncoronary branch arteries, including the carotid and vertebral, upper extremity, visceral, and lower extremity arterial branches. According to the American Heart Association, PAD is the preferred clinical term used to describe disease of the arteries of the arms and legs. This chapter focuses on lower extremity PAD, although it is recognized that upper extremity PAD, especially subclavian artery stenosis, can be an important clinical disorder that may lead to discrepant blood pressures in the arms, arm claudication, or vertebral–subclavian or coronary–subclavian steal phenomena. In addition, while lower extremity PAD may uncommonly be caused by nonatherosclerotic disease (e.g., large vessel vasculitis, arterial entrapment syndromes, fibromuscular dysplasia), this chapter focuses on lower extremity PAD due to atherosclerotic vascular disease, the most common cause.
EPIDEMIOLOGY AND RISK FACTORS
The prevalence of PAD increases with age, and the disease affects men and women nearly equally, up to 29% of the elderly population in a general medical practice.2,3 Risk factors for PAD are similar to those of coronary artery disease. They can be categorized as hereditary or acquired. The most important risk factors for PAD are advanced age, diabetes mellitus, and tobacco use. Additional risk factors for PAD are shown in Table 50.1.
TABLE
50.1 Risk Factors for Lower Extremity PAD

PAD IS A MARKER OF INCREASED CARDIOVASCULAR RISK
PAD is a marker for extensive systemic atherosclerosis and high cardiovascular risk. It has been estimated that among patients with symptomatic PAD (claudication) the 5-year mortality is as high as 30% with an additional 20% suffering a nonfatal MI or stroke.4 In published case series of PAD patients, 60% to 80% have significant coronary artery disease in at least one vessel on angiography5,6 and up to 25% of patients will have significant internal carotid artery stenosis 7,8 In the recent REACH registry, 21% of patients with PAD suffered an MI, a stroke, cardiovascular death, or hospitalization within 1 year of follow-up as compared to 15% of patients with established coronary artery disease.9
A low ABI has been shown in multiple studies to be an independent predictor of mortality.10,11 Patients with ABI of <0.90 are twice as likely to have a history of MI, angina, and heart failure than patients with an ABI of >1.0.12,13 An abnormal ABI also provides complimentary information to Framingham risk score (FRS) and increases cardiovascular risk prediction. A low ABI (≤0.90) was associated with approximately twice the 10-year total mortality, cardiovascular mortality, and major coronary event rate compared to the overall rate in each FRS category.14 In the recently published German Epidemiological Trial on Ankle-Brachial Index (GET ABI), an abnormal ABI for both symptomatic and asymptomatic patients was associated with increased risk of major cardiovascular events.15
NATURAL HISTORY OF PAD
Less than 5% of patients with stable claudication will require amputation;4 however, the annual risk increases dramatically to 30% to 40% among those with critical limb ischemia (CLI).16 It has been demonstrated that patients with PAD have significant function and QOL impairment. The QOL impairment among PAD patients is similar to that of patients with congestive heart failure or recent MI.17 Objective evidence of clinical depression is twice as common among patients with PAD.18 PAD leads to functional impairment with decreased walking distance and speed in patients with claudication and even among patients with atypical leg symptoms.19 Such functional impairment can diminish a patient’s abilities to work, exercise regularly, and participate in recreational activities.
The classic symptom of lower extremity PAD is claudication, which is derived from Latin and meaning to limp. Claudication is defined as recurrent burning, aching, fatigue, or heaviness in the leg muscles that is provoked with a predictable level of exercise and that resolves with a predictable duration of rest (generally <10 minutes). The Rose claudication questionnaire is a simple screening tool for claudication that can be used in clinical practice by asking the patient two simple questions: “Do you get pain in either leg when you walk?” and “Does the pain go away when you stop walking?” If the answer to both questions is yes, the likelihood of PAD is >95%.20 Claudication must be distinguished from other potential diagnoses, including pseudoclaudication due to lumbar canal stenosis that can present with buttock, back, and thigh pain with both exertion and prolonged standing. While claudication is an important symptom of PAD, it is important to recognize that the majority of patients with PAD do not present with classic intermittent claudication (Fig. 50.1). The majority of patients with PAD will exhibit leg discomforts that do not meet the definition of claudication (atypical leg symptoms), while others remain completely asymptomatic and are identified only through measures such as the ABI.
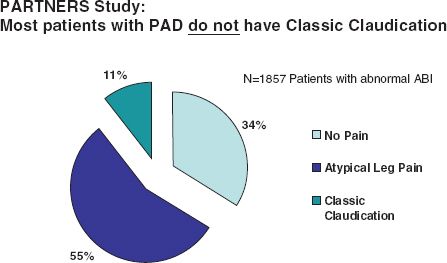
FIGURE 50.1 Clinical presentation of lower extremity PAD in the stable patient. In these data from the PARTNERS study, the majority of patients identified to have an abnormal ABI on a screening examination were either asymptomatic or had leg symptoms atypical for claudication. Only 11% in this study had classic intermittent claudication. These findings emphasize the variable clinical presentation of PAD and the need to incorporate modalities beyond screening for claudication (such as the ABI) to diagnose this disease. (Adapted from Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–1324.)
Acute Limb Ischemia and Critical Limb Ischemia
There are two severe clinical presentations of lower extremity PAD that must be clinically recognized in a timely fashion. Acute limb ischemia, characterized by the “6Ps” of pain, paresthesias, pallor, pulselessness, paralysis, and poikilothermia (or “polar”), is a vascular emergency due to thrombotic arterial occlusion that requires urgent revascularization with thrombolytic therapy and/or thrombectomy. CLI is a more common severe clinical presentation of PAD. CLI is defined as objectively proven PAD plus ischemic rest pain, nonhealing ulceration, or gangrene that has been present for at least 2 weeks.21 The most important risk factors for development of CLI among patients with stable PAD are diabetes mellitus and ongoing tobacco use, but older patient age (>65 years), lower baseline ABI, and hyperlipidemia also increase risk.21 CLI is vascular urgency that requires expedient evaluation for revascularization. Patients with CLI are at a very high risk of a major cardiovascular event in the year following diagnosis, including death, and careful assessment of cardiovascular risk factor control and symptoms is important in this population.
Physical Examination Findings in Peripheral Arterial Disease
A comprehensive history and vascular examination is key for diagnosis and appropriate management of PAD. The comprehensive physical examination in the PAD patient should include measurement of blood pressure in bilateral arms (to screen for subclavian stenosis/upper extremity PAD); assessment of the carotid, upper extremity, and lower extremity (femoral, popliteal, dorsalis pedis [DP], posterior tibial) pulses; and assessment of the abdominal aorta. Using standardized definitions, pulses are graded on a scale from 0 to 3 with 2 = normal pulse, 1 = diminished pulse, 0 = nonpalpable pulse, and 3 = abnormally bounding or aneurysmal pulse.16 If no pulse is palpable, Doppler examination using a handheld continuous-wave device should be performed. An absent posterior tibial pulse has high specificity for diagnosis of PAD. Abnormalities of the DP pulse are less specific due to a high prevalence of anomalous or absent DP arteries in healthy patients. In addition to pulse deficits, other physical examination findings consistent with lower extremity PAD include vascular bruits (heard over femoral or popliteal arteries), hair loss, nail hypertrophy, and rapid elevation pallor or dependent rubor of the leg. Socks must be removed at every visit to carefully inspect the feet for evidence of tissue loss (i.e., ulcers or gangrene) as well as for signs of concomitant foot pathology, such as peripheral neuropathy or excessive callous formation.
DIAGNOSTIC TESTING FOR PAD
Ankle–Brachial Index
The ABI is the ratio of ankle systolic pressure to brachial systolic pressure and is determined by measuring systolic blood pressures in bilateral brachial arteries and at bilateral DP and posterior tibial arteries using a handheld Doppler device. Calculation of the ABI is shown in Figure 50.2. Interpretation guidelines for the ABI are shown in Table 50.2. The range of normal values for an ABI is 1.0 to 1.4. A normal resting ABI does not always rule out PAD. If the ABI is borderline (e.g., 0.91 to 0.99) or normal and the clinical suspicion of PAD is high, repeat ABI after treadmill exercise should be performed. An ABI may fall by 20% or more in patients with significant PAD after exercise. This is especially true among patients with aortoiliac disease (“inflow disease”).
Figure 50.2 Calculation of the ABI. The ABI is the ratio of blood flow in the legs to the arms. The ankle blood pressure for each limb is the higher of the DP and posterior tibial artery pressures. For both the right and left ABI calculation, the higher of the two arm blood pressures is used for the denominator. (From Cleveland Clinic Foundation, with permission.)
TABLE
50.2 Interpretation of the ABIa
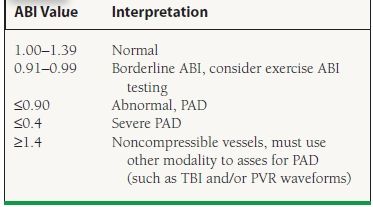
aABI values are interpreted according to the 2011 update of the American College of Cardiology Foundation/American Heart Association Guideline for the management of Patients with Peripheral Artery Disease. Rooke TW, et al. J Am Coll Cardiol. 2011;58(19):2020–2045.
It should also be emphasized that an ABI of >1.4 is not normal and suggests noncompressible vessels either from vascular calcification (“medial calcinosis”) or from inability to compress arteries due to obesity. This is commonly seen among patients with diabetes and chronic kidney disease. A high or falsely elevated ABI has been shown to confer increased cardiovascular risk in epidemiologic studies.10,14 In clinical practice, an ABI > 1.4 cannot be interpreted and thus cannot confirm or rule out the diagnosis of PAD without another diagnostic test, such as pulse volume recordings (PVRs), the toe–brachial index (TBI), or an imaging modality.
Imaging Studies for PAD
Beyond the ABI, segmental leg pressures with PVRs or Doppler tracings may be used to localize disease by anatomic segments (e.g., aortoiliac disease, femoropopliteal disease, infrapopliteal disease). Transcutaneous oximetry may be helpful to determine tissue perfusion and limb viability in the setting of CLI and ulceration. In some cases, imaging modalities may be indicated to more definitively define anatomy and establish severity of disease. Diagnostic modalities for PAD are shown in Table 50.3. In general, imaging studies are most appropriately reserved for selected patients with PAD, such as for revascularization planning or for postprocedural graft or stent surveillance or for cases in which the diagnosis of PAD or the nature of disease is uncertain. For example, these imaging studies are particularly important for evaluation of nonatherosclerotic causes of PAD (e.g., arterial aneurysm, fibromuscular dysplasia, entrapment syndromes, and vasculitis). Imaging studies may also be indicated to establish the diagnosis of PAD in the setting of noncompressible vessels (i.e., ABI > 1.4).
TABLE
50.3 Diagnostic Modalities for PAD
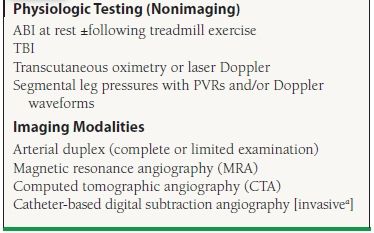
aAngiography is generally reserved for cases in which revascularization is anticipated or noninvasive testing is inadequate to comprehensively evaluate the patient.
MANAGEMENT OF PAD: A THREE-PRONGED APPROACH
The comprehensive care of the PAD patient must address three important aspects: protection of the feet and prevention of limb loss, prevention of cardiovascular events, and improvement of functional capacity and QOL (Fig. 50.3).
FIGURE 50.3 The three-pronged approach to PAD care. Comprehensive care of the PAD patient must include all three aspects of care. (From Cleveland Clinic Foundation, with permission.)
Foot Care and Ulcer Prevention
The feet of the PAD patient must be examined at every office visit. The importance of meticulous foot and nail care should be reviewed regularly, including the need for regular foot self-inspection, moisturization of the feet, and use of appropriate footwear. Patients should be educated regarding the symptoms and signs of CLI and advised to seek medical attention should these develop. Patients should be referred for podiatric care when indicated, and the use of diabetic footwear and orthotic devices should be considered for patients with diabetes mellitus, foot deformities, or excessive callous formation.
Prevention of Cardiovascular Events
Therapies to prevent MI and stroke are crucial for every patient with PAD, including those without known atherosclerotic carotid or coronary artery disease. While there are little data regarding the benefits of CV risk reduction therapies among asymptomatic patients with an abnormal ABI, the same therapies are generally recommended for these PAD patients. Elements of a risk reduction program for the PAD patient are shown in Table 50.4 and discussed in detailed in both the American College of Cardiology/American Heart Association (ACC/AHA) and the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC-II) guidelines.16,21,21a Cardiovascular risk reduction therapies are underprescribed among patients with PAD and in comparison to those with coronary artery disease.22,23
TABLE
50.4 Therapies to Prevent Cardiovascular Events Among Patients with PAD
Smoking Cessation
It is well established that smoking cessation decreases the risk of MI, stroke, and malignancy and improves survival. Among patients with PAD, smoking cessation has been shown (in epidemiologic studies) to lower the risk of amputation, need for revascularization, and bypass graft failure and to improve overall survival.24–27 Asking patients about their smoking status at every visit and counseling them to quit is the first step in the management of PAD. Pharmacotherapy for smoking cessation such as nicotine replacement, bupropion, and varenicline should be considered and offered. The use of varenicline, a partial α4β nicotinic acetylcholine receptor agonist, has shown to be effective in smokers with cardiovascular disease, including PAD.28 Referring patients to a formal smoking cessation program, when available, may be helpful.
Antiplatelet Agents
Clinical trials and meta-analyses have shown that antiplatelet medications like aspirin are efficacious in secondary prevention for coronary artery disease and carotid disease. Antiplatelet therapy reduces the incidence of major vascular events among symptomatic PAD patients by approximately 25% as shown in a large meta-analysis.29 Based upon these data, both the ACC/AHA and TASC II PAD guidelines recommend antiplatelet therapy with either aspirin or clopidogrel for patients with PAD.16,21,21a However, the optimal agent and dose of antiplatelet therapy for patients with PAD have not been definitively established. Data from the prevention of progression of arterial disease and diabetes (POPADAD) trial and Aspirin for Asymptomatic Atherosclerosis (AAA) trial demonstrated a lack of benefit of low-dose aspirin (100 mg/d) for prevention of cardiovascular events among patients with asymptomatic PAD and borderline abnormal ABI.30,31 The CLIPS trial demonstrated a significant benefit of aspirin 100 mg/d versus placebo among patients with symptomatic or asymptomatic PAD with mean ABI = 0.63, although this study was stopped early due to poor recruitment and had some methodologic flaws.32 A recently published meta-analysis of trials of aspirin for symptomatic and asymptomatic PAD patients has shown a need for additional studies to confirm the benefit of aspirin for CV risk reduction in PAD patients.33 When clopidogrel was compared with aspirin among high-risk patients with atherosclerotic vascular disease in the CAPRIE study (Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events),33 there was a small but incremental benefit of clopidogrel, particularly among the subgroup of symptomatic PAD patients.34 A focused update of the 2005 ACC/AHA PAD guidelines has been published that addresses these new antiplatelet data.21a Antiplatelet therapy with either aspirin or clopidogrel is recommended for patients with lower extremity PAD for reduction of cardiovascular events.21a Given a lack of compelling efficacy data, dual antiplatelet therapy is not recommended for PAD patients unless there is another compelling indication such as recent acute coronary syndrome or coronary stenting.35
Warfarin
Warfarin in combination with antiplatelet medication was compared with antiplatelet medication alone in PAD patients in the WAVE trial (Warfarin Antiplatelet Vascular Evaluation). The combination was not more beneficial but was associated with an increased risk of significantly life-threatening bleeding.36 Based upon these data, warfarin is not recommended unless there is another compelling indication for its use (e.g., venous thromboembolism or atrial fibrillation).16,21a
Lipid-Lowering Therapy
All patients with PAD should be treated with an HMG coenzyme-A reductase inhibitor (statin), unless there is a compelling contraindication. The Heart Protection Study randomized high-risk and relatively normocholesterolemic patients (total cholesterol ≥135 mg/dL) to simvastatin and placebo, including 4,588 patients with lower extremity PAD.37 Statins were associated with a 13% reduction in all-cause mortality, 17% reduction in cardiovascular mortality, and 24% reduction in first major vascular event.37 Among the subset of PAD patients, randomization to simvastatin was associated with a 20% reduction in noncoronary revascularization procedures.38 Statin therapy has been shown to improve claudication symptoms and slowed the rate of functional decline among patients with PAD.39,40 The 2005 ACC/AHA PAD guidelines recommend statin therapy to a goal low-density lipoprotein (LDL) cholesterol < 100 mg/dL for PAD patients (Class I, level of evidence [LOE] B) with an option to target cholesterol to <70 mg/dL for very high risk patients (Class IIa, LOE B). For PAD patients with low highdensity lipoprotein (HDL) cholesterol and/or significantly elevated triglycerides, additional lipid-lowering agents (e.g., niacin and fibrates) may be considered, but generally as adjunctive therapy to statins.
Blood Pressure Control
Current practice guidelines recommend antihypertensive therapy for hypertensive PAD patients to achieve a goal of <140/90 mm Hg for nondiabetic patients or of <130/80 mm Hg for PAD patients with concomitant diabetes mellitus or chronic renal disease.16 Clinical trials have further established the benefit of more intensive blood pressure control, particularly with the angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers. The HOPE trial included 4,046 patients with symptomatic PAD among its population of patients with atherosclerotic vascular disease or diabetes and other risk factors and compared ramipril verses placebo for CV risk reduction among relatively normotensive patients. The trial showed a 22% reduction in major CV events among patients randomized to ramipril.41 The ONTARGET trial established a role for angiotensin receptor blockers (telmisartan) as an alternative to ACE inhibitors in the management of CV risk in patients with atherosclerotic vascular disease, including PAD.42 In this study, the combination of ramipril plus telmisartan showed no incremental benefit and increased the risk of significant adverse events including hypotension and hyperkalemia compared to the individual therapies. Intensive blood pressure control among diabetic patients with PAD is particularly important. The ABCD trial compared intensive blood pressure control to standard therapy among diabetic patients using nisoldipine or enalapril. In this trial, intensive BP control essentially eliminated the inverse relationship of ABI and adverse cardiovascular events43 (Fig. 50.4).
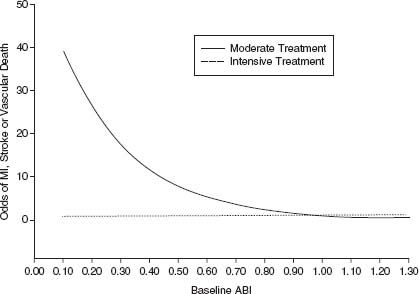
FIGURE 50.4 Intensive blood pressure control reduces risk of cardiovascular events among diabetic patients with PAD: Data from the ABCD trial. Intensive blood pressure control with nisoldipine or enalapril (dashed line) essentially eliminated the relationship of reduced ABI and risk of a major cardiovascular event that was seen in the standard therapy arm (solid line). (Reproduced from Mehler PS, Coll JR, Estacio R, et al. Intensive blood pressure control reduces the risk of cardiovascular events in patients with peripheral arterial disease and type 2 diabetes. Circulation. 2003;107(5):753–756, with permission.)




