Patients with heterotaxy syndrome (HS) have a range of anomalies and outcomes. There are limited data on perinatal outcomes after prenatal diagnosis. To determine the factors influencing perinatal and infant outcomes, we analyzed prenatal and postnatal variables in fetuses with HS from 1995 to 2011. Of 154 fetuses with HS, 61 (40%) had asplenia syndrome (ASP) and 93 (60%) had polysplenia syndrome (PSP). In the ASP group, 22 (36%) patients were elected for termination of pregnancy, 4 (10%) had fetal death, and 35 of 39 (90%) continued pregnancies were live born. In the PSP group, 12 (13%) patients were elected for termination of pregnancy, 5 (6%) had fetal death (4 with bradyarrhythmia), and 76 of 81 (94%) continued pregnancies were live born. Bradyarrhythmia was the only predictor of fetal death. In the live-born ASP group, 43% (15 of 35) died, 7 because of pulmonary vein stenosis, 4 postoperatively, and 4 because of noncardiac causes. In the live-born PSP group, 13% (10 of 76) died, 5 postoperatively, 2 from bradyarrhythmia, 1 from a cardiac event, and 2 from noncardiac causes. Pulmonary vein stenosis and noncardiac anomalies were independent risk factors for postnatal death. Only 8% of ASP patients achieved biventricular circulation, compared with 65% of PSP patients. In the live-born cohort, the 5-year survival rate was 53% for ASP and 86% for PSP. In conclusion, most PSP patients are currently alive with biventricular circulation in contrast with few ASP patients. Bradyarrhythmia was the only predictor of fetal death. Pulmonary vein stenosis and noncardiac anomalies were predictors of postnatal death.
Heterotaxy syndrome (HS) encompasses a broad range of cardiac and noncardiac anomalies with outcomes that range from favorable, with minimal disease burden over a lifetime, to the worst end of the spectrum, with defects that can be lethal in utero, in the neonatal period, and in childhood. Moreover, in those patients who require palliative surgery for their complex congenital heart disease, many will later succumb to the burden of the Fontan circulation. Pediatric studies on HS have demonstrated cardiac risk factors that determine outcomes. However, there are limited data on perinatal outcomes after prenatal diagnosis of HS and risk factors for death. Studying the fetal cohort is important as an increasing percentage of congenital heart defects are diagnosed prenatally. Furthermore, although prenatal diagnosis of HS has not been shown to affect postnatal survival, it likely affects pregnancy outcome and allows for informative counseling and, in the perinatal period, alerts the medical team to institute appropriate medical care in a timely fashion. Additionally, unlike most patients with congenital heart disease who are stable throughout gestation, patients with HS have the potential to have a compromised cardiovascular status, in particular from bradyarrhythmia with the often-associated cardiomyopathy. In HS, prenatal cardiac anatomic and physiologic characteristics that are risk factors for death have not been well elucidated. Because of the rarity of the disease, current data consist of relatively small patient series. Therefore, we sought to do a retrospective analysis of a larger cohort of patients prenatally diagnosed with HS in a more recent surgical era to determine factors influencing perinatal outcomes and those influencing short-term postnatal outcomes.
Methods
We included fetuses with HS diagnosed at Boston Children’s Hospital from 1995 to 2011. Patients were identified in the fetal echocardiogram database. The diagnosis of HS was determined by the previously well-described criteria, and patients were further categorized as those with asplenia syndrome (ASP) (right atrial isomerism) or polysplenia syndrome (PSP) (left atrial isomerism).
For the purposes of analysis, the cohort was analyzed in 2 time periods: during gestation and during the postnatal period. Clinical status, current circulation (univentricular vs biventricular), and reoperations at most recent follow-up were obtained from the medical record or from the primary referring cardiologist.
We collected maternal demographic and pregnancy-related data and data on fetal structural cardiac defects. Patients with mitral stenosis or atresia, aortic stenosis or atresia, and those with aortic arch obstruction were analyzed as having left heart obstruction. Patients with tricuspid stenosis or atresia and those with pulmonary stenosis or atresia were analyzed as having right heart obstruction. The presence of bradyarrhythmia, such as sinus bradycardia (heart rate <100 beats/min) or complete atrioventricular (AV) block (atrial rate higher than ventricular rate with complete dissociation between the 2), was studied. Noncardiac diagnoses (structural and genetic) were also analyzed. The prenatal diagnosis of the heart defects was confirmed after birth by echocardiography, and in cases of fetal death and termination of pregnancy, it was confirmed by autopsy, when available.
In the postnatal cohort of prenatally diagnosed patients with HS, we collected data on cardiac and noncardiac diagnoses, and surgical procedures were performed. Patients receiving antibiotic prophylaxis were categorized as having immune dysfunction.
The primary outcome was death, including termination of pregnancy, spontaneous fetal death, or postnatal death. Postnatal death was described as early (death before discharge after initial hospitalization) or late (death after discharge of initial hospitalization or at >30 days of age). Secondary outcomes were the need for cardiac surgical procedures and type of circulation achieved.
Review of the medical records was approved by the Boston Children’s Hospital Institutional Review Board. Patient characteristics and outcomes were summarized as number (percent) for categorical variables and median (interquartile range) for continuous variables without normal distribution. Comparison among groups was performed with 2-sample t test in variables with normal distribution or Wilcoxon rank sum test in those without normal distribution. The Kaplan-Meier method was used to estimate survival probabilities. Cox proportional hazard regression model was used to estimate hazard ratios with 95% confidence intervals. Values of p <0.05 were considered statistically significant.
Results
Our cohort included 154 fetuses with HS, of whom 61 (40%) were categorized as ASP patients and 93 (60%) as PSP patients. Mean maternal age at diagnosis was 28.8 ± 5.8 years and the diagnosis of HS by fetal echocardiography was made at a median gestational age of 22 weeks (19 to 30). Gestational age at diagnosis was significantly lower in those not live born, which included termination of pregnancy and spontaneous fetal death (20 vs 26 weeks, p <0.0001). The anatomic cardiac findings of each group are listed in Table 1 . Univentricular-type defects were more frequent in the ASP group (75% vs 20%, p <0.0001). Of note, there were 25 patients in the PSP cohort with interrupted inferior vena cava, abdominal heterotaxy, and normal intracardiac anatomy.
| Variable | Asplenia Syndrome (n = 61) | Polysplenia Syndrome (n = 93) |
|---|---|---|
| Systemic venous anomalies | ||
| Interrupted IVC | 5 (8%) | 69 (74%) |
| Bilateral SVC | 18 (29%) | 16 (17%) |
| Pulmonary venous anomalies | ||
| TAPVC | 28 (46%) | 0 |
| PAPVC | 0 | 9 (10%) |
| Atrioventricular canal abnormalities | ||
| Complete AVC defect | 39 (64%) | 14 (15%) |
| Partial or transitional AVC | 0 | 4 (4%) |
| Ventricular abnormalities | ||
| Hypoplastic LV/Single RV | 5 (8%) | 5 (5%) |
| Hypoplastic RV/Single LV | 38 (62%) | 10 (11%) |
| Single ventricle, uncertain morphology | 0 | 2 (2%) |
| Ventriculoarterial abnormalities | ||
| Double outlet RV | 32 (52%) | 6 (6%) |
| Transposition of great arteries | 1 (2%) | 1 (1%) |
| Malposed great arteries | 0 | 3 (3%) |
| Tetralogy of Fallot | 0 | 2 (2%) |
| Truncus arteriosus | 1 (2%) | 0 |
| Ventricular outflow obstruction | ||
| Pulmonary/subpulmonary obstruction | 44 (72%) | 5 (5%) |
| Aortic/subaortic obstruction | 7 (11%) | 2 (2%) |
| Coarctation of aorta | 2 (3%) | 4 (4%) |
| Isolated atrial septal defect | 0 | 3 (3%) |
| Isolated ventricular septal defect | 1 (2%) | 5 (5%) |
| Atrioventricular valve regurgitation ∗ | 4 (7%) | 3 (3%) |
| No significant intracardiac abnormality † | 0 | 25 (27%) |
∗ Moderate/severe regurgitation.
Of the ASP patients, 36 of 61 (59%) were diagnosed before 24 weeks of gestation, of whom 22 of 36 (61%) were elected for termination of pregnancy. Eighty percent of the ASP patients who elected for termination of pregnancy had univentricular-type cardiac defects, and half of those also had totally anomalous pulmonary venous connections (TAPVCs). Of those with ASP in continued pregnancies (39 of 61), 4 (10%) had fetal death: 1 patient with right heart obstruction and an intraventricular mass, 1 with Ebstein anomaly and hydrops, and 1 with AV canal and bilateral outflow tract obstruction. The fourth patient with AV canal and single ventricle died of an unknown cause. The remaining 35 of 39 (90%) patients who continued the pregnancy were live born ( Figure 1 ).
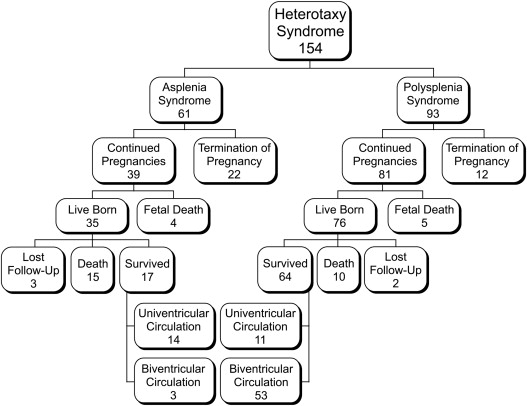
Of the PSP patients, 61 of 93 (66%) were diagnosed before 24 weeks of gestation, of whom 12 of 61 (20%) were elected for termination of pregnancy. Of those, 5 of 12 patients had univentricular-type cardiac defects and 7 had biventricular-type cardiac defects, of whom 3 had associated bradyarrhythmia. Of the 81 of 93 patients who continued the pregnancy, 5 (6%) had fetal death: 4 with bradyarrhythmia and 1 with multiple anomalies. The remaining 76 of 81 (94%) patients were live born.
Prenatal sinus bradycardia or AV block was exclusive to PSP patients (22 of 93, 24%). Of these, 13 of 22 (59%) had sinus bradycardia at diagnosis, and 9 of 22 (41%) had complete AV block. In the 22 fetuses with bradyarrhythmia, 15 of 22 (68%) survived to birth, 3 of 22 (14%) had elective termination of pregnancy, and 4 of 22 (18%) had fetal death. Of those with fetal death, 2 had AV block and 2 had sinus bradycardia. No fetal treatment was administered for the bradyarrhythmia. Excluding termination of pregnancy, 15 of 19 fetuses (79%) survived to birth. At birth, 4 patients had sinus bradycardia and 11 had complete AV block confirmed by electrocardiogram postnatally. Despite pacemaker implantation in the neonatal period, 3 patients died in infancy, all with complete AV block. Of 15 live-born patients, 12 (80%) were alive at recent follow-up, 9 of them requiring pacemaker placement. Further details for this subgroup of patients have been published recently by our group.
Abdominal situs abnormalities were present in 26 of 61 (43%) ASP patients and in 51 of 93 (55%) PSP patients. Overall, 8 patients were found to have genetic anomalies in addition to HS (3 of them diagnosed prenatally). After birth, we routinely performed upper gastrointestinal series, abdominal ultrasound, and splenic uptake scans in all HS patients. The prevalence of noncardiac anomalies diagnosed after birth was higher in the ASP group (27 of 35, 77% vs 42 of 76, 55%, p <0.05), with intestinal malrotation being the most common finding ( Table 2 ). In the total cohort, ∼50% of patients were on antibiotic prophylaxis. There was a significant difference between groups with ASP more likely to be on long-term antibiotic prophylaxis (81% in ASP vs 37% in PSP, p <0.001).
| Anomalies | Asplenia Syndrome n = 35 | Polysplenia Syndrome n = 76 |
|---|---|---|
| Total | 27 (77%) | 42 (55%) |
| Gastrointestinal anomalies | ||
| Malrotation | 21 (60%) | 26 (34%) |
| Ladd procedure | 7 (20%) | 9 (12%) |
| Biliary atresia | 0 | 3 (4%) |
| Duodenal atresia | 0 | 1 (1%) |
| Omphalocele | 1 (3%) | 1 (1%) |
| Genetic defects | ||
| Trisomy 21 | 0 | 2 (3%) |
| Trisomy 18 | 1 (3%) | 0 |
| Rubinstein-Taybi syndrome | 0 | 1 (1%) |
| Prader-Willi syndrome | 0 | 1 (1%) |
| Kartagener syndrome | 0 | 1 (1%) |
| CNS anomalies | 1 (3%) | 2 (3%) |
| Others | ||
| Diaphragmatic hernia | 1 (3%) | 1 (1%) |
| Cleft palate | 0 | 2 (3%) |
| Vertebral fusion | 0 | 1 (1%) |
| Agenesis ductus venosus | 1 (3%) | 0 |
| Cystic adenomatoid malformation | 0 | 2 (3%) |
| Congenital deafness | 1 (3%) | 0 |
In the live-born cohort of ASP, 15 of 35 (43%) patients have died. There were 5 early deaths: 3 patients died in the neonatal period after cardiac surgery and 2 died after the parents elected for comfort care (1 patient with trisomy 18, and 1 patient with single ventricle and TAPVC). There were 10 late deaths: 7 patients (7 of 15, 47%) died because of pulmonary vein stenosis (6 of 7 with TAPVC) at a median age of 20 months (5 to 46) and 2 others as a result of noncardiac problems (1 with volvulus and intestinal necrosis and 1 with encephalitis), both of them were on antibiotic prophylaxis. One patient had a sudden cardiac death. Three patients were lost to follow-up. Starting with fetal diagnosis and excluding termination of pregnancy, 17 of 39 (44%) ASP patients are currently alive with a median follow-up of 10 months (range 4 days to 15.3 years).
In the live-born cohort of PSP, 10 of 76 (13%) patients have died. There were 8 early deaths: 6 patients died after cardiac surgery and 2 because of AV block and prematurity. There were 2 late deaths because of sepsis and multiorgan failure; both these patients were on antibiotic prophylaxis. Two patients were lost to follow-up. Starting with fetal diagnosis and excluding termination of pregnancy, 64 of 81 (79%) PSP patients are currently alive with a median follow-up of 4.3 years (range 0 to 14).
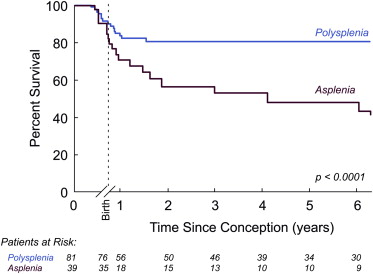
In the live-born cohort, the 5-year survival rate was 53% for ASP and 86% for PSP ( Figure 2 ).
Of the patients who were alive at the end of the study period, all 17 ASP patients have undergone cardiac surgery compared with 35 of 64 (55%) PSP patients (p <0.001). There was a significant difference in the ultimate circulation achieved between the 2 groups: 3 of 17 (17%) patients with ASP achieved a biventricular circulation, compared with 53 of 64 (83%) PSP patients (p <0.0001). After excluding PSP patients who did not require surgery, 28 of 64 (44%) patients achieved a biventricular circulation ( Figure 1 ).
Of the fetuses with ASP and continued pregnancies, only 3 of 39 (8%) are currently alive with biventricular circulation. In contrast, of those with PSP, 53 of 81 (65%) are currently alive with a biventricular circulation.
The presence of bradyarrhythmia was the only risk factor for fetal death in the PSP group (odds ratio = 16.3, 95% confidence interval [CI] 1.7 to 156, p = 0.002). Those fetuses with bradyarrhythmia died at a mean gestational age of 24.5 ± 3.4 weeks. In ASP, we did not find any specific risk factor associated with fetal death. Variables associated with postnatal death in univariate analysis are listed in Table 3 . After multivariate regression analysis, only the presence of pulmonary vein stenosis was a strong predictor of postnatal death (HR = 5.6, 95% CI [2.3 to 13.9], p <0.001). When excluding patients with normal hearts, pulmonary vein stenosis (HR = 7.7, 95% CI [2.8 to 21.3], p <0.001) and noncardiac anomalies (HR = 4.5, 95% CI [1 to 20.2], p = 0.04) were independent predictors of death. There was no statistical difference in postnatal mortality during the first and the second half of the study (24% vs 21%, p = 0.8). In univariate analysis, when analyzing ASP and PSP groups separately, TAPVC (HR = 3.6, 95% CI [1.2 to 11], p = 0.02) and pulmonary vein stenosis (HR = 3, 95% CI [1.0 to 8.7], p = 0.04) were associated with postnatal death in ASP group. In this group of ASP, 14 of 15 live-born patients with TAPVC underwent surgical repair. Nine have died, 6 of them (6 of 14, 43%) from pulmonary vein stenosis. In PSP group, univentricular circulation (HR = 3.8, 95% CI [1.1 to 13.6], p = 0.03), moderate/severe AV valve regurgitation (HR = 17.5, 95% CI [1.9 to 157.2], p = 0.01), and left heart obstruction (HR = 6.4, 95% CI [1.8 to 22.4], p = 0.004) were associated with postnatal death.



