Fig. 10.1
Stepwise parsimonious approach to diagnosing the etiology of pericardial effusion
Simple clinical assessment has been shown to assist in establishing the diagnosis: large effusion without ‘inflammatory’ signs or clinical signs of tamponade (jugular venous distention, hypotension, and/or pulsus paradoxus) commonly signifies chronic idiopathic pericardial effusion (likelihood ratio = 20, P < 0.001) whereas large effusions with clinical signs of tamponade and without ‘inflammatory’ signs should raise the suspicion for malignancy (likelihood ratio = 2.9, P < 0.001) [9]. Pericardial effusion sampling for diagnostic purposes and occasionally pericardial biopsy should be considered in following settings:
1.
Concern for purulent and tuberculous pericarditis;
2.
Clinical suspicion of neoplastic pericardial effusion;
3.
Moderate to large pericardial effusion in patients with advanced HIV and/or immune suppression;
4.
Moderate to large or progressive pericardial effusion in patients that are not responding to initial therapy or when the tiered work-up is inconclusive.
Pericardial fluid analysis can include Gram and acid-fast bacilli stains and cultures, polymerase chain reaction, tuberculosis-specific testing (e.g., adenosine deaminase, lysozyme, and gamma-interferon), tumor markers, and cytology [2]. Contrary to common practice and unlike pleural effusion work-up, cell count, lactate dehydrogenase and protein and glucose levels have not been shown to be particularly useful in differential diagnosis and management of patients with pericardial effusion [12]. Pericardioscopy allows a targeted pericardial biopsy and it can potentially increase the diagnostic accuracy of sampling (e.g., neoplastic pericardial effusion) [13].
Assessing Hemodynamic Significance of Pericardial Effusion
When evaluating the hemodynamic impact of pericardial effusion one should take into account the acuity of presentation. Acute accumulation of fluid (within minutes to hours) rapidly exceeds the pericardial stretch limit and commonly presents as cardiogenic shock [14]. This dramatic presentation is called acute or surgical tamponade and it requires immediate intervention. Chamber perforation during a percutaneous procedure is a good example of acute tamponade. Blunt chest trauma and ascending aortic dissection resulting in blood accumulation within pericardium require prompt surgical intervention and percutanous pericardial effusion drainage is relatively contraindicated [2]. When pericardial fluid accumulates slowly (within days to weeks) a large amount of fluid can be present without dramatic lowering of the cardiac output [14]. This can lead to subacute or medical tamponade which requires careful assessment of both clinical and imaging data to establish the need for pericardial effusion drainage [15]. The following discussion will elaborate the assessment for subacute (medical) tamponade.
History and Physical Examination
Although many refer to pericardial tamponade as a “clinical diagnosis,” the existing evidence suggests that subacute tamponade is a difficult diagnosis to make on mere clinical grounds. Dyspnea is the cardinal symptom of subacute pericardial tamponade, but it is very nonspecific [16]. Other symptoms such as fever, cough, and chest pain can occur and typically reflect the underlying cause (i.e., pericarditis) rather than pericardial fluid accumulation. Clinical findings of pericardial tamponade include tachycardia, jugular venous distention, pulsus paradoxus, and diminished heart sounds and all lack both sensitivity and specificity. Tachycardia is common in hospitalized patients for many reasons and it could be blunted by medications such as beta-blockers. In a systematic review, the jugular venous distention had a pooled sensitivity of 76 % for cardiac tamponade [16]. Assessment of jugular venous distention is limited by the experience of the observer; it can be difficult in some patients, even for experienced clinicians [17, 18]. Besides, jugular venous distention is associated with other conditions causing shortness of breath such as pulmonary hypertension and congestive heart failure. While patients with acute (surgical) tamponade rapidly progress to cardiogenic shock, hypotension is rather uncommon in patients with subacute tamponade who accumulate pericardial effusion within days to weeks. On the contrary, many patients are hypertensive due to high levels of circulating catecholamines in response to hemodynamic stress [19, 20]. In studies of pericardial tamponade, the mean systolic blood pressure ranged from 127–144 mmHg [19]. According to a recent review, hypertensive tamponade is seen in 27–43 % of patients [19]. Systolic blood pressure commonly decreases in these patients after pericardial effusion drainage, and treating the hypertensive response without draining the effusion can be dangerous [19, 21].
Pulsus Paradoxus
Pulsus paradoxus is considered the cornerstone of the clinical diagnosis of pericardial tamponade [14]. Interestingly, it is not a “paradoxical” phenomenon but an exaggeration of the physiologic decrease in systolic arterial pressure with inspiration. Under normal conditions, the decrease in blood pressure is <10 mmHg, and it is explained by phasic variation in the filling of the right- and left-sided cardiac chambers related to intrathoracic pressure changes with respiration. With tamponade, the accumulating pericardial effusion restricts cardiac filling and makes the respiratory variation in the right and left ventricular filling more pronounced and interdependent [14]. Pulsus paradoxus is measured by manual sphygmomanometer as the difference between intermittent and persistent Korotkoff sounds during normal respiration, not with deep breathing (Fig. 10.2) [16]. A wide variation in the incidence of pulsus paradoxus has been reported in patients with pericardial tamponade, ranging from 12 to 75 % [22]. According to one study, approximately 20 % of tamponade patients had “low-pressure” cardiac tamponade defined as low intrapericardial pressure and low post-drainage right atrial pressure [23]. In “low-pressure” tamponade patients, the incidence of jugular venous distention was 22 % and pulsus paradoxus was reported in only 7 % of patients [23]. Besides limited sensitivity for pericardial tamponade, pulsus paradoxus is not very specific. A myriad of conditions have been reported to be associated with pulsus paradoxus; a short list includes asthma, right ventricular infarction, severe hypovolemia, constrictive pericarditis, restrictive cardiomyopathy, pneumothorax, chronic obstructive lung disease, and pulmonary embolism [15]. Some of these conditions can also cause jugular venous distention and tachycardia, common associated findings of pericardial tamponade.
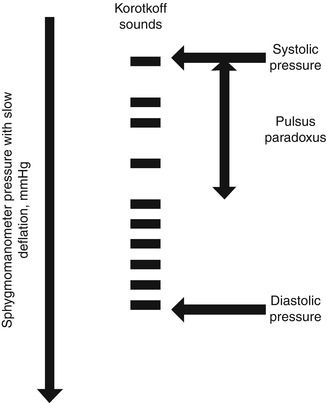

Fig. 10.2
Obtaining pulsus paradoxus as the difference between intermittent and persistent Korotkoff sounds during normal respiration
Invasive and Imaging Data
Before the widespread use of echocardiography invasive data using cardiac catheterization were commonly obtained to confirm the diagnosis of tamponade. Cardiac catheterization in tamponade demonstrates equilibration of diastolic intracardiac pressures and respiratory variation in right and left-sided cardiac pressures corresponding to pulsus paradoxus [2].
Echocardiography is currently the cornerstone of hemodynamic evaluation of pericardial effusion [2]. Normally, intrapericardial pressure is lower than the central venous pressure. As pericardial fluid accumulates the intrapericardial pressure equilibrates first with the right sided filling pressures and then left-sided filling pressures [16]. During tamponade, intrapericardial pressure may temporarily exceed intracavitary pressure in various chambers during cardiac cycle and result in chamber collapse. Certain pitfalls should be kept in mind when interpreting echocardiographic findings. Transient buckling of the right atrium is commonly seen in patients with pericardial effusion and it is not specific [24]. A more sustained collapse of the right atrium lasting at least one third of the cardiac cycle appears to be more specific for cardiac tamponade [24]. Right ventricular early diastolic collapse is a less sensitive finding but has a high specificity. Right ventricular outflow tract should be inspected carefully for the signs of collapse since it is the thinnest area of the right ventricle and M-mode echocardiography should be used for precise timing. Left-sided chamber collapse is much less sensitive but highly specific for tamponade [2]. Importantly, a study by Merce et al. showed that 34 % of patients with pericardial effusion but without clinical features of pericardial tamponade had at least one chamber collapse on echocardiography [25]. Therefore, in patients with pericardial effusion who have chamber collapse, one should carefully document respiratory flow variation across valves as a sign of ventricular interdependence and interrogate the inferior vena cava size and collapsibility as a sign of elevated right-sided filling pressures (Fig. 10.3) [26]. An abnormal filling pattern in the superior vena cava with a markedly diminished diastolic flow can also be observed. We define respiratory variation in the inflow velocities conservatively as >30 % across mitral valve and >60 % for tricuspid valve [11]. These echocardiographic signs, when present, increase the specificity of diagnosis [26]. Finally, the size of pericardial effusion seems to be an important but frequently underappreciated part of the echocardiographic assessment. In one study of hospitalized patients with pericardial effusion, the size of the effusion was the only independent predictor of adverse in-hospital outcomes in a multivariate model, but not chamber collapse or inferior vena cava plethora [27]. We define a moderate pericardial effusion as the largest pocket between 1 and 2 cm at the end diastole and a large effusion as >2 cm [1].
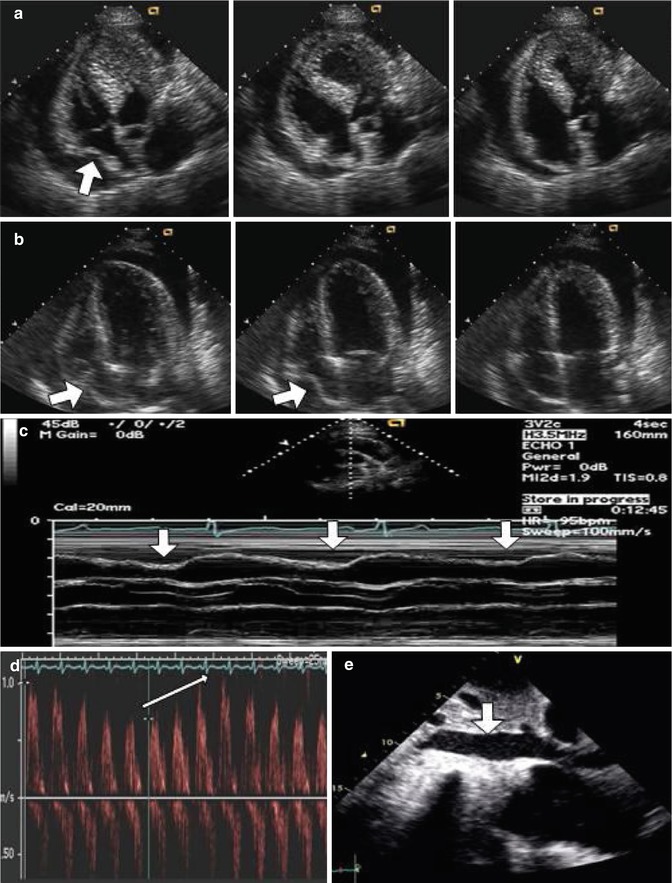

Fig. 10.3
Echocardiographic findings in pericardial tamponade. (a) Transient inward motion of the right atrium (arrow) is commonly seen in patients with pericardial effusion and it is not specific for tamponade. (b) A sustained collapse of the right atrium (arrows) lasting at least one third of the cardiac cycle is specific for tamponade. (c) M-mode echocardiography showing diastolic collapse of the right ventricular outflow tract (arrows) is specific for tamponade. (d) Respiratory variation in the mitral inflow velocities (arrow) and (e) vena cava engorgement (arrow) are supportive signs for cardiac tamponade
The diagnosis of cardiac tamponade may be particularly difficult in patients with pulmonary hypertension and right ventricular failure because they commonly accumulate pericardial effusion. Pericardial effusion in these patients is a marker of adverse outcomes [28]. Common clinical findings of pericardial tamponade such as tachycardia and jugular venous distention may not be helpful in differential diagnosis for shortness of breath and progressive right-sided heart failure. Collapse of the left-sided cardiac chambers has been described as an important echocardiographic clue to the presence of cardiac tamponade in these settings [29]. Conversely, more common findings of tamponade such as right atrial and ventricular collapse can be masked by elevated right-sided filling pressures. Poor outcomes have been reported with routine draining of pericardial effusion in these patients [30].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


