25 Pericardial Effusion
I. CASE
A. Fetal echocardiography findings
1. Fetal echocardiography reveals situs solitus of the atria, levocardia, left aortic arch, and heart rate of 142 bpm.
2. The four-chamber view is normal. Cardiac axis and position are normal. Size is at the upper limits of normal (cardiothoracic ratio = 0.34). Ventricles are of equal size.
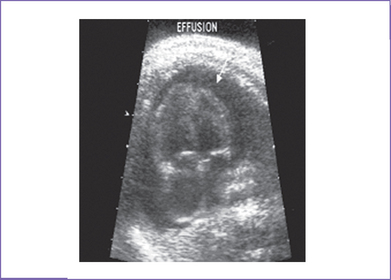
3. There is a small circumferential pericardial effusion measuring 4 mm in diameter at the mitral valve level (Fig. 25-1). At the tricuspid valve level, the effusion is 6 mm in diameter.
4. The branch pulmonary arteries are a good size. The aortic annulus is of normal size with increased (1.3 m/s) Doppler velocity through it.
5. There is trace to mild tricuspid regurgitation (a holosystolic jet) of mild severity.
6. The aortic arch is leftward and the ductal and aortic arches are of similar size. Both arches have antegrade flow.
7. Atrial flow is normal right to left, and there is a normal pulmonary venous flow pattern.
8. The right ventricle (RV) and left ventricle (LV) Tei indices (myocardial performance index) are normal.
9. The middle cerebral artery peak systolic velocity is 0.75 m/s, which is elevated, suggesting anemia.
10. Peripheral Doppler is normal, including ductus venosus.
11. M-mode echocardiogram of the atrial and ventricular walls shows no diastolic collapse.
12. Ventricular fractional shortening is 45% in the LV and 30% in the RV.
D. Fetal management and counseling
1. Because isolated pericardial effusions in utero can be associated with aneuploidy, amniocentesis was offered but was declined.
2. Follow-up included serial antenatal studies every week.
a. Size of effusion, possible cardiac tamponade (atrial or ventricular diastolic collapse).
b. Signs of heart failure as evidenced by abnormal venous Doppler, tricuspid valve or mitral valve regurgitation or abnormal ventricular shortening fraction, increased heart size, and abnormal arterial Doppler for placental function (see cardiovascular profile score in Chapter 30).
c. Biophysical profile for signs of fetal distress.
d. Serial assessment for fetal anemia (middle cerebral artery peak velocity) and potential benefit of fetal transfusion.
e. The possibility of fetal myocarditis should be considered if myocardial function parameters (e.g., fractional shortening, Tei index) are abnormal.
E. Delivery
1. Location of the delivery depends upon the size of the pericardial effusion and potential for compromise, the presence of additional pathology, and the risk of missed diagnosis of significant ventricular aneurysm or diverticulum or of ductal occlusion.
2. In isolated pericardial effusion, vaginal delivery (unless obstetrically contraindicated) should be at term or as near term as possible in a tertiary care center.
3. If the effusion is associated with hydrops and low cardiovascular score profile, then assisted induced delivery in a tertiary care center should be considered. Certainly, with a larger effusion or with any evidence of evolving hydrops with or without a definitive etiology, delivery in a tertiary care center should be considered.
4. Pericardiocentesis immediately before delivery may be warranted in the context of a very large or hemodynamically important pericardial effusion to assist in the resuscitation after birth of the neonate.
F. Neonatal management
1. Echocardiography should be performed for effusion size, more subtle evidence of tamponade, and evaluation of cardiac structure and function.
2. Blood work should be obtained to exclude fetal or neonatal infection and hematologic abnormalities such as anemia that might be associated with a pericardial effusion.
3. If signs of heart failure are present (tachypnea, tachycardia, hepatomegaly, and cardiomegaly), then anticongestive medications may be indicated.
G. Follow-up
1. Follow-up depends upon confirmation after birth of a persistent pericardial effusion.
2. The baby should be evaluated at a cardiology clinic visit in 1 week after discharge.
3. If there is any history of unexplained palpitations or abnormal heart rhythm, a 24-hour Holter monitoring should be considered.
4. One or two yearly outpatient follow-up visits should be scheduled with 12-lead electrocardiogram (ECG) and echocardiogram to exclude cardiomyopathy, which is rare but has been reported.
I. Outcome of this case
1. Maternal serologic and molecular biology polymerase chain reaction (PCR) techniques were done at the time of the diagnosis of a pericardial effusion because transplacental parvovirus infection was suspected. The tests confirmed parvovirus infection.
2. Because there was mild cardiomegaly and no other evidence of significant anemia with only mildly increased middle cerebral Doppler indices, normal ductus venosus flow, and no evidence of progressive hydrops, the fetus was serially assessed by general ultrasound and fetal echocardiography, and no prenatal intervention was considered necessary.
3. The baby was born at term with good Apgar scores and good weight for gestation.
4. Postnatal echocardiography showed mild cardiomegaly and small pericardial effusion with normal biventricular function.
5. The baby’s full blood count showed mild anemia and thrombocytopenia.
6. PCR and IgM (immunoglobulin M) titers confirmed a diagnosis of parvovirus.
7. The baby was asymptomatic and was discharged home after 2 days of observation. There was no sign of arrhythmia.
8. At follow-up, gradual resolution of the pericardial effusion, cardiomegaly, and anemia was seen.
II. YOUR HANDY REFERENCE
A. Pericardial effusion
a. A small amount of pericardial fluid during routine prenatal ultrasound screening is not uncommon, being observed in 44% of normal fetuses.
b. In some reports it has been shown that in low-risk gestations and in the absence of other ultrasound abnormalities, an isolated effusion of up to 7 mm has not been associated with poor fetal outcome.
2. Etiology of pericardial effusion.
a. Idiopathic (particularly common in the context of small pericardial effusions).
b. As part of an evolving picture of hydrops fetalis, which itself has a large differential diagnosis.
c. Associated with aneuploidy and syndromes (presumably due to abnormal lymphatics in most cases).
d. Associated with an inflammatory process or metabolic abnormality.
e. Associated with intrapericardial teratoma.
f. Associated with right or left ventricular apical aneurysms, which are usually associated with massive pericardial effusions, at least early in their course.
3. In one study, the outcome and associations of 35 consecutive cases of isolated pericardial effusion detected in the fetus were presented.
a. In all cases included in the study, there was no evidence of a structural abnormality or a rhythm disturbance detectable antenatally.
b. Karyotyping revealed that 26% of cases had trisomy 21 and 31% of the total had some form of chromosomal anomaly.
c. This study showed that the outlook for isolated pericardial effusion is good.
4. Parvovirus may be the cause of as much as one third of all incidents of hydrops fetalis. Outcome in such fetuses is good; spontaneous resolution occurs in approximately one third of such incidents, and approximately 85% of those who receive fetal transfusions survive. The virus is not teratogenic and, despite reports of viral persistence in myocardial and brain tissues, neurodevelopmental outcome in survivors appears to be normal.
5. Associated syndromes and extracardiac anomalies.
a. Some studies have shown the high incidence of associated karyotypic anomalies, in particular trisomy 21.
b. Fetal karyotyping is therefore recommended in patients with isolated pericardial effusion.
6. Clues to fetal sonographic diagnosis.
a. Pericardial effusion is easily detected on echocardiogram. An anechoic region larger than 2 mm separating the pericardial layer may be observed. A small rim of pericardial effusion may be normal.
b. Usually, pericardial effusion is seen close to the atrioventricular (AV) valve or to one of the ventricles, rarely around the whole heart. The myocardial periphery can be confused with minimal pericardial fluid due to the presence of circular fibers, which gives an anechoic quality to this region (pericardial sweat).
c. Color Doppler in the pericardial effusion has a characteristic appearance, with to and fro movements. Pulsed Doppler can be used to confirm the velocity changes in the effusion. If blood is suspected in the pericardium, MRI may be useful in confirming this rare finding.
d. Middle cerebral artery Doppler measurements can be made to assess the hematocrit noninvasively and help to identify other causes of fetal anemia.
7. Cardiovascular profile score.
a. Pericardial effusion deducts 1 point from the cardiovascular profile score.
b. In anemia and arteriovenous fistula patients, it is important to evaluate the valve function by Doppler. With arteriovenous fistula, surveillance for valvar regurgitation is indicated because this is an early sign of cardiac decompensation.
c. In intrapericardial teratoma there should be close evaluation for signs of increased central venous pressure. It has been suggested that venous Doppler assessment may be helpful in identifying the fetus at risk for progressive hydrops.
a. Small pericardial effusions alone do not usually compromise the fetus.
b. An enlarging pericardial effusion can lead to hemodynamic compromise of the fetus as a result of cardiac tamponade. The walls of the atria or ventricle might collapse and the ventricular filling patterns might be altered, with development of an E/A wave ratio that approaches 1 or even a dominant E wave.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree