10
Pericardial Disease
Pericardial Anatomy and Physiology
The pericardium consists of two serous surfaces surrounding a closed, complex, saclike potential space. The visceral pericardium is continuous with the epicardial surface of the heart. The parietal pericardium is a dense but thin fibrous structure that is apposed to the pleural surfaces laterally and blends with the central tendon of the diaphragm inferiorly. Around the right and left ventricles (RV and LV) and the ventricular apex, the pericardial space is a simple ellipsoid structure conforming to the shape of the ventricles. Around the systemic and pulmonary venous inflows and around the great vessels, the parietal and visceral pericardia meet to close the “ends” of the sac—these areas often are referred to as pericardial reflections. The pericardial space encloses the right atrium (RA) and RA appendage anteriorly and laterally, with pericardial reflections around the superior and inferior vena cavae near their junction with the RA. Superiorly, the pericardium extends a short distance along the great vessels, with a small “pocket” of pericardium surrounding the great arteries posteriorly—the transverse sinus. The pericardial space extends laterally to the left atrium (LA), and a blind pocket of the pericardium extends posteriorly to the LA, between the four pulmonary veins—the oblique sinus (Fig. 10-1). The pericardial space normally contains a small amount (5 to 10 mL) of fluid that may be detectable by echocardiography.
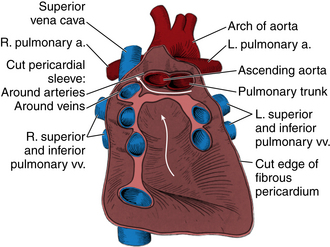
Figure 10–1 Pericardial anatomy.
The posterior wall of the pericardial sac after the heart has been removed by severing its continuity with the great arteries and veins and by cutting the two pericardial sleeves that surround the arteries and veins. The parietal serous pericardium is dark red, the fibrous pericardium is pink, the horizontal arrow is in the transverse sinus, and the vertical arrow is in the oblique sinus of the pericardium. (Reprinted with permission from Rosse C, Goddum-Rosse P: Hollinshead’s Textbook of Anatomy, 5th ed. Philadelphia: Lippincott-Raven, 1997.)
Pericarditis
Pericarditis is inflammation of the pericardium, and it can be due to a wide variety of causes, including bacterial or viral infection, trauma, uremia, and transmural myocardial infarction (Table 10-1). Clinically, the diagnosis of pericarditis is based on at least two of the four characteristic features:
TABLE 10-1
Causes of Pericardial Disease (with Examples)
Idiopathic
Infections
Viral
Bacterial (Staphylococcus, pneumococcus, tuberculosis)
Parasitic (Echinococcus, amebiasis, toxoplasmosis)
Malignant
Metastatic disease (e.g., lymphoma, melanoma)
Direct extension (e.g., lung carcinoma, breast carcinoma)
Primary cardiac malignancy
Inflammatory
Post-myocardial–infarction (e.g., Dressler’s syndrome)
Uremia
Systemic inflammatory diseases (e.g., lupus, scleroderma)
Post-cardiac surgery
Radiation
Intracardiac-Pericardial Communications
Blunt or penetrating chest trauma
Postcatheter procedures
Postinfarction LV rupture
Aortic dissection
Echocardiographic Approach
In a patient with suspected pericarditis, the echocardiogram may show a pericardial effusion of any size, pericardial thickening with or without an effusion, or it may be entirely normal. A pericardial effusion is recognized as an echolucent space around the heart (Fig. 10-2).
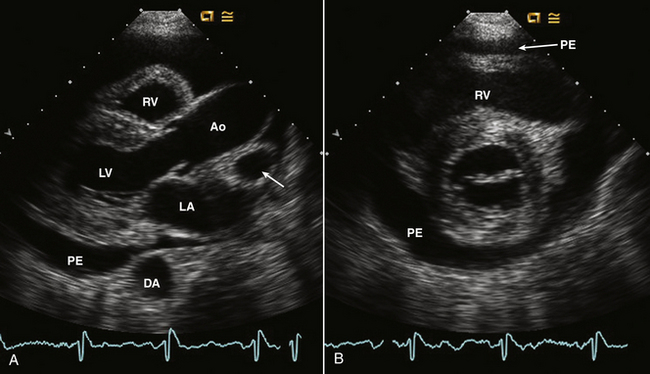
Figure 10–2 ![]() Pericardial effusion on echocardiography.
Pericardial effusion on echocardiography.
Parasternal long- and short-axis views of a moderate circumferential pericardial effusion (PE). In the long-axis view (A) and short-axis view (B), the effusion tracks appear anterior to the descending aorta (DA) with a small amount of fluid posterior to the LA in the oblique sinus. Pericardial fluid in the transverse sinus (posterior to the aorta [Ao]) delineates the right pulmonary artery (arrow) which is not usually seen in this view in adults. Pericardial fluid anterior to the RV is seen in both the long- and short-axis views.
Pericardial thickening is evidenced by increased echogenicity of the pericardium on two-dimensional (2D) imaging and as multiple parallel reflections posterior to the LV on M-mode recordings (Fig. 10-3). However, because the pericardium typically is the most echogenic structure in the image, it can be difficult to distinguish normal from thickened pericardium, and other imaging approaches, such as computed tomography (CT) or magnetic resonance (CMR), are more sensitive for this diagnosis.
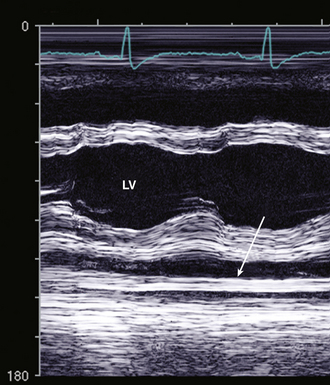
Figure 10–3 Pericardial thickening on M-mode echocardiography.
Multiple parallel dense echos (arrow) are seen posterior to the LV epicardium. This patient also has a small pericardial effusion (PE), seen on M-mode as an echo-free space between the flat pericardium and moving posterior wall.
Pericardial Effusion
A wide variety of disease processes can result in a pericardial effusion with a differential diagnosis similar to that for pericarditis (see Table 10-1). The physiologic consequences of fluid in the pericardial space depend both on the volume and rate of fluid accumulation. A slowly expanding pericardial effusion can become quite large (>1000 mL) with little increase in pericardial pressure, whereas rapid accumulation of even a small volume of fluid (50 to 100 mL) can lead to a marked increase in pericardial pressure (Fig. 10-4).
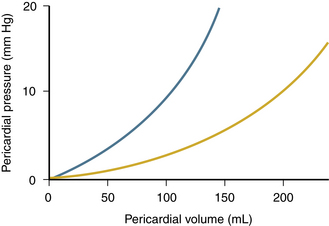
Figure 10–4 Pericardial pressure versus pericardial volume.
The graph shows an acute effusion (blue line, with a steep pressure-volume relationship) and a chronic effusion (yellow line, where large volumes may lead to only mild pressure elevation).
Tamponade physiology occurs when the pressure in the pericardium exceeds the pressure in the cardiac chambers, resulting in impaired cardiac filling (Fig. 10-5). As pericardial pressure increases, filling of each cardiac chamber is sequentially impaired, with lower-pressure chambers (atria) affected before higher-pressure chambers (ventricles). The compressive effect of the pericardial fluid is seen most clearly in the phase of the cardiac cycle when pressure is lowest in that chamber—systole for the atrium, diastole for the ventricles. Filling pressures become elevated as a compensatory mechanism to maintain cardiac output. In fully developed tamponade, diastolic pressures in all four cardiac chambers are equal (and elevated) because of exposure of the entire heart to the elevated pericardial pressure.
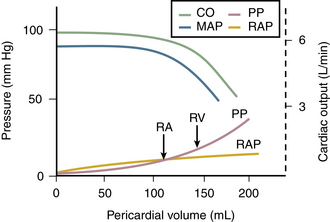
Figure 10–5 Relationship among pericardial pressure (PP), RA pressure (RAP), mean arterial pressure (MAP), and cardiac output (CO).
Note that when pericardial pressure exceeds RA pressure, blood pressure and cardiac output fall. When RV pressure is exceeded (at the arrow), cardiac output and mean arterial pressure fall further.
Clinically, tamponade physiology manifests as lowcardiac output symptoms, hypotension, and tachycardia. Jugular venous pressure is elevated and pulsus paradoxus (an inspiratory decline >10 mm Hg in systemic blood pressure) is present on physical examination. The clinical finding of pulsus paradoxus is closely related to the echo findings of reciprocal respiratory changes in RV and LV filling and emptying.
Diagnosis of Pericardial Effusion
Diffuse Effusion
A pericardial effusion is recognized as an echolucent space adjacent to the cardiac structures. In the absence of prior pericardial disease or surgery, pericardial effusions usually are diffuse and symmetric with clear separation between the parietal and visceral pericardium (Fig. 10-6). A relatively echogenic area anteriorly, in the absence of a posterior effusion, most likely represents a pericardial fat pad. M-mode recordings are helpful, especially with a small effusion, showing the flat posterior pericardial echo reflection and the moving epicardial echo with separation between the two in both systole and diastole.
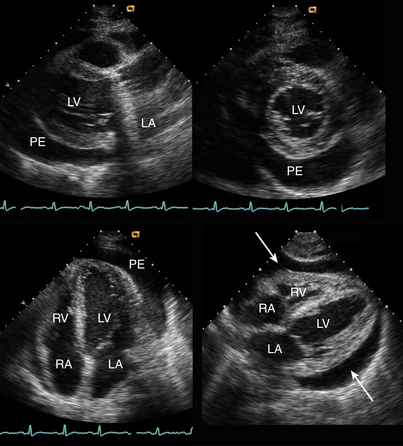
Figure 10–6 ![]() Circumferential pericardial effusion.
Circumferential pericardial effusion.
The echolucent effusion (PE) is seen in parasternal long-axis, short-axis, apical four-chamber, and subcostal views in a patient early after mechanical aortic valve replacement. Note the shadowing and reverberations from the valve in the parasternal long-axis view.
In patients with recurrent or long-standing pericardial disease, fibrinous stranding within the fluid and on the epicardial surface of the heart may be seen. When a malignant effusion is suspected, it is difficult to distinguish this nonspecific finding from metastatic disease. Features suggesting the latter include a nodular appearance, evidence of extension into the myocardium, and the appropriate clinical setting (Fig. 10-7).
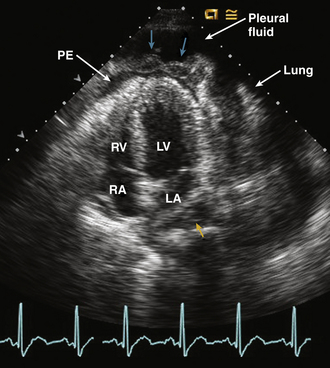
Figure 10–7 Malignant pericardial effusion.
Apical four-chamber view in a patient with metastatic lymphoma shows a small pericardial effusion (PE) in the apical region with marked thickening and irregularity of the pericardium (cyan arrows), suggesting tumor involvement. Pleural fluid with compressed lung also is evident. The small fluid collection adjacent to the LA (yellow arrow) may be pericardial fluid in the oblique sinus of the pericardium.
Loculated Effusion
After surgical or percutaneous procedures, or in patients with recurrent pericardial disease, pericardial fluid may be loculated (Fig. 10-8). In this situation, the effusion is localized by adhesions to a small area of the pericardial space or consists of several separate areas of pericardial effusion, separated by adhesions. Recognition of a loculated effusion is especially important because hemodynamic compromise can occur with even a small, strategically located fluid collection. In addition, drainage of a loculated effusion may not be possible from a percutaneous approach.
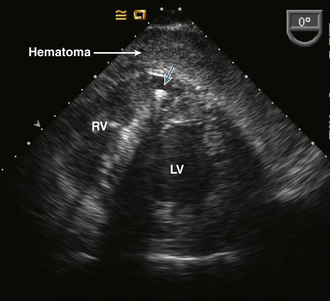
Figure 10–8 Pericardial hematoma
TEE transgastric short-axis view in a patient with acute hypotension during an electrophysiology procedure shows a localized hematoma in the pericardial space with compression of the RV. The catheter in the RV (cyan arrow) casts a dark shadow that obscures part of the ventricular septum.
Distinguishing from Pleural Fluid
In order to reliably exclude the possibility of a loculated pericardial effusion, echocardiographic evaluation requires examination from multiple acoustic windows. The parasternal approach demonstrates the extent of the fluid collection at the base of the heart in both long- and short-axis views. Note that pericardial fluid may be seen posterior to the LA (in the oblique sinus), as well as posterior to the LV. Care should be taken that the coronary sinus or descending thoracic aorta is not mistaken for pericardial fluid. In fact, these structures can help in distinguishing pericardial from pleural fluid, because a left pleural effusion will extend posterolaterally to the descending aorta, whereas a pericardial effusion will track anterior to the descending aorta (Fig. 10-9). When a large left pleural effusion is present, sometimes cardiac images can be obtained with the transducer on the patient’s back (Fig. 10-10).
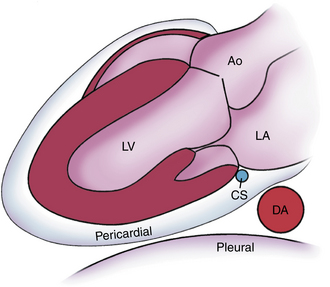
Figure 10–9 ![]() Pericardial versus pleural fluid.
Pericardial versus pleural fluid.
Schematic diagram of the relationship between a pericardial effusion and the descending aorta (DA) compared with a left pleural effusion. Pericardial fluid tracks posterior to the LA in the oblique sinus of the pericardium, anterior to the descending aorta. Ao, aorta; CS, coronary sinus.
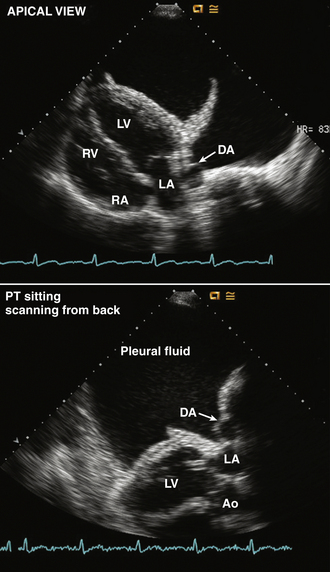
Figure 10–10 Large pleural effusion.
In a view with the transducer moved laterally from the apical position (top), a large left pleural effusion is seen. This can be distinguished from pericardial fluid by the position of the descending aorta (DA), the presence of compressed lung, and by identification of both layers of the pericardium adjacent to the myocardium. Images also were obtained with the transducer on the patient’s back (bottom), demonstrating the relationship between the pleural fluid and the descending aorta.







