I. Introduction.
The pericardium is a fibrous sac composed of two layers. The inner monocellular visceral layer is composed of mesothelial cells and is adherent to the myocardium. The outer parietal layer is a fibrous layer < 2 mm thick that consists mostly of collagen and elastin. It is joined to adjacent intrathoracic structures by means of ligaments. Interspersed between the two layers is a small amount of serous pericardial fluid, generally about 15 to 35 mL. The normal pericardium is distensible and permits unimpeded expansion of the ventricles during diastole. Normally, changes in intrathoracic pressure are easily transmitted to the heart, resulting in increased venous return to the right side of the heart with inspiration and increased pulmonary venous return to the left side of the heart with expiration.
The pericardium helps to maintain the position of the heart within the chest cavity, acts to reduce friction during the cardiac cycle, and also acts as a barrier to infection and inflammation. The pericardium secretes prostaglandins that can modulate cardiac reflexes and coronary tone.
A. Acute pericarditis is a clinical syndrome caused by inflammation of the pericardium, and it is associated with chest pain, a friction rub, and characteristic electrocardiographic changes. The incidence of acute pericarditis is 2% to 6% in autopsy series, although it is diagnosed clinically in only 1 in 1,000 admissions. It is more common in adults (20 to 50 years of age) and in men.
B. Constrictive pericarditis is caused by fibrous thickening of the pericardium secondary to chronic inflammation from a variety of causes.
C. Pericardial effusion is a fluid collection in the pericardial space. The clinical presentation may range from being asymptomatic to life-threatening hemodynamic compromise, depending on the underlying cause of the effusion and the rate of accumulation, as discussed later in detail.
D. Cardiac tamponade is a clinical emergency that arises when a pericardial fluid collection impairs diastolic filling sufficiently to produce a low cardiac output state.
II. ACUTE PERICARDITIS.
There are a large number of potential etiologies of acute pericarditis. In practice, these are classified into the following groups: idiopathic, infectious, inflammatory, uremic, post-myocardial infarction (post-MI), neoplastic, and traumatic (
Table 40.1).
A. Etiology
1. Idiopathic. Most cases of acute pericarditis are idiopathic, although many of these may be viral in origin.
2. Viral pericarditis. The most commonly involved viruses are coxsackievirus B and echovirus. A prodrome of upper respiratory tract symptoms preceding the onset of chest pain, along with a fourfold or higher rise in viral convalescent antibody titers, supports the diagnosis. Most cases are self-limited; infrequent complications include myocarditis (i.e., myopericarditis), recurrent pericarditis, pericardial effusion, tamponade, and constrictive pericarditis.
3. Purulent pericarditis. Purulent pericarditis usually occurs as a complication of pneumonia or empyema caused by staphylococci, pneumococci, or other streptococci. Early diagnosis of purulent pericarditis is paramount, as cardiac tamponade often develops and is associated with high mortality. Purulent pericarditis is characterized by acute onset of fever, shaking chills, night sweats, and dyspnea of a few days duration. Chest pain or pericardial friction rub is not necessarily present.
4. Tuberculous pericarditis. Although uncommon in the United States, this entity should be considered in patients with fever and pericardial effusion, particularly if there is an underlying immunocompromised state. Pericardial involvement occurs in 1% to 2% of cases of pulmonary tuberculosis. If the clinical suspicion is high, the patient should be hospitalized and started on triple drug therapy, while definitive diagnostic testing is undertaken (acid-fast bacilli [AFB] testing and pericardial/pleural biopsy).
5. Post-MI pericarditis occurs most often after a large anterior wall MI. Because post-MI pericarditis is a marker for extensive myocardial necrosis, these patients are at an increased risk for congestive heart failure and mortality at 1 year after MI. It is notable that the rate of post-MI pericarditis has declined since the introduction of successful reperfusion therapies.
6. Dressler’s syndrome usually occurs weeks to several months after MI, with an incidence of about 1%. It presents as malaise, fatigue, and chest pain that can be of concern for recurrent MI. The cause of Dressler’s syndrome is unclear, although it has been proposed to be autoimmune in nature.
7. Postpericardiotomy syndrome. Although similar to Dressler’s syndrome in presentation, it usually occurs within the first 6 to 8 weeks following cardiac surgery. The incidence varies from 10% to 40%, and the syndrome is believed to be caused by an autoimmune reaction.
8. Uremic pericarditis typically develops in patients who are just beginning renal replacement therapy with hemodialysis. The majority present with a rub, and the associated pericardial effusions tend to be large. The cause is unknown but does not seem to be related to the level of circulating uremic catabolites or toxins.
9. Neoplastic pericarditis. Tumors involving the pericardium are typically metastatic in nature (lung, breast, Hodgkin’s and non-Hodgkin’s lymphoma, and leukemia). It is important to suspect cardiac tamponade in patients with a known malignancy who present with symptoms of relatively acute onset fatigue, dyspnea, or edema.
10. Autoimmune and inflammatory. Lupus, rheumatoid arthritis, vasculitis, and other rheumatologic disorders are also associated with pericarditis.
B. Clinical presentation
1. Signs and symptoms
a. Chest pain from pericarditis is described as a severe, sharp retrosternal pain that may radiate to the neck, shoulders, and back, worsening when lying supine, coughing, or during inspiration. The pain may be alleviated when the patient leans forward.
b. There may be a prodrome of fever and myalgias.
c. Dyspnea may result from shallow breathing due to inspiratory chest pain.
d. Patients with purulent pericarditis may appear toxic with high fevers, shaking chills, and night sweats.
e. Tuberculous pericarditis is characterized by gradual onset of symptoms with chronic, nonspecific, constitutional symptoms such as fever, chills, and night sweats.
2. Physical findings
a. The pericardial friction rub is the major clinical finding in pericarditis, but it is not present in all cases. It is described as a scratchy, grating, and high-pitched sound. The rub is often evanescent, changes in quality and intensity on serial examinations, and may be accentuated with deep respiration. Classically, it has three components, corresponding to atrial systole, ventricular systole, and early ventricular diastole. Most often, however, it is a biphasic rub consisting of the atrial and ventricular systolic components.
b. Auscultation of the rub is ideally performed using the diaphragm of the stethoscope at the left lower sternal border during inspiration, with the patient leaning forward.
C. Laboratory examination and diagnostic testing. Pericarditis is a clinical diagnosis based on history, physical examination, chest radiograph, and serial electrocardiographic changes. Based on the clinical scenario, some patients may require further testing, such as tuberculin skin testing, fungal tests, viral serologies, cold agglutinins, thyroid function tests, heterophile antibodies, antinuclear antibodies, rheumatoid factor, bacterial culture, and cytology.
1. Electrocardiography. The associated electrocardiographic changes evolve through
four stages (
Table 40.2). Although these changes occur in most patients, their
absence does not exclude acute pericarditis, particularly in patients with neoplastic or tuberculous pericarditis.
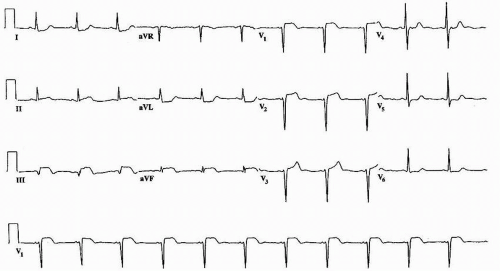
a. The first stage usually occurs within hours of the onset of chest pain and is diagnostic of acute pericarditis (
Fig. 40.1). The presence of
stage 1 electrocardiographic changes is most useful in confirming the diagnosis of acute pericarditis, yet such changes are often
difficult to distinguish from changes associated with early repolarization and acute infarction. There is
diffuse ST-segment elevation with upright T waves in all leads
except aVR and V
1.
PR-segment depression is seen in all leads except aVR and V
1. There is often PR-segment elevation in lead aVR (the “knuckle” sign).
b. Stage 2, which occurs several days later, is characterized by resolution of PR/ST segments to baseline and T-wave flattening.
c. The T-wave inversions mark stage 3.
d. Stage 4 occurs when T waves become upright again, which may take days to weeks.
e. With a large effusion, the electrocardiogram (ECG) may show electrical alternans or low voltage.
2.A chest radiograph may reveal cardiomegaly and may yield important information in support of tuberculous or neoplastic processes.
3. Blood cultures along with sputum and gastric aspirate for tuberculosis should be done where such a diagnosis of purulent or tuberculous pericarditis is suspected (including in immunosuppressed immigrants). Pericardial or pleural biopsy may be necessary to diagnose tuberculosis.
4. Blood tests may reveal leukocytosis or an elevated erythrocyte sedimentation rate, which are nonspecific markers of inflammation. Mild elevations in the creatine kinase myocardial band (CK-MB) fraction or cardiac troponin levels can be seen and suggest a more extensive acute inflammatory process involving the epicardium; significant elevations in these markers should raise suspicion for more extensive myocardial involvement, referred to as myopericarditis.
5. Echocardiography
a. Pericarditis is not an echocardiographic diagnosis and a normal echocardiogram does not preclude pericarditis. Echocardiography should be performed when symptoms last longer than a week, to evaluate for hemodynamic abnormalities.
b. If the patient has had recent cardiac surgery, is elderly, or if there is a suspicion for pericardial effusion, echocardiography should be done as part of the initial workup.
6. Computed tomography (CT), magnetic resonance imaging (MRI), or transesophageal echocardiography (TEE) can be done in select cases for further investigation of the pericardium (refer to
Chapters 52,
51, and
68, respectively, for detailed discussions on these modalities).
E. Therapy.
Most cases of acute pericarditis are uncomplicated and self-limited and such patients can be managed in the outpatient setting. However, inpatient management should be considered in patients with large pericardial effusion or coexisting myocarditis. These typically respond to conservative medical therapy. First-line therapy usually consists of nonsteroidal anti-inflammatory drugs (NSAIDs) with the addition of colchicine in some cases.
1. Medical therapy
a. Ibuprofen has a good safety profile and is a reasonable first-line therapy at doses of 600 to 800 mg orally three times a day for at least 2 weeks. Aspirin 650 mg orally every 6 to 8 hours for 2 to 4 weeks is an alternative therapy. Other NSAID agents, including naproxen, seem to be similarly efficacious.
b.If the
patient does not respond to NSAIDs, or in cases of recurrent pericarditis,
colchicine should be considered in addition to NSAIDs. A randomized controlled trial, colchicine for acute pericarditis (COPE), of 120 patients
with a first episode of acute pericarditis demonstrated superior efficacy of a combined regimen of aspirin with colchicine. The usual dosing of colchicine is 1 to 2 mg for the first day and then 0.5 to 1 mg daily for 3 months.
c. Prednisone should be used only in patients with recurrent pericarditis with persistent symptoms despite NSAIDs and colchicine therapy or in cases where there is an underlying inflammatory disease that is responsive to corticosteroid therapy. Prednisone should be dosed at 1 to 1.5 mg/kg for at least 1 month and should be tapered slowly. In the COPE trial, corticosteroid therapy was an independent risk factor for recurrence.
d. Post-MI pericarditis patients should not be treated with prednisone, given the risk of myocardial rupture. Treatment with aspirin is recommended (650 mg every 6 hours).
e. In cases of suspected purulent pericarditis, empiric antibiotic therapy directed against staphylococci and streptococci should be instituted while cultures are pending.
f.For tuberculous pericarditis, standard triple drug therapy is recommended for at least 9 months, with 6 months of treatment following culture conversion.
g. Pericarditis due to Dressler’s syndrome should be managed with NSAIDs or aspirin. If the condition is recurrent, a trial of prednisone may be warranted.
h. Intensive dialysis is the treatment of choice for symptomatic uremic pericarditis. Dialysis is not necessary for patients who are asymptomatic with relatively small pericardial effusions.
2. Percutaneous therapy
a.Because most cases of pericarditis are self-limited, there is no role for routine pericardiocentesis, intrapericardial administration of steroids, or pericardial biopsy.
b. In cases complicated by tamponade or suspected purulent effusion or neoplasm, pericardiocentesis should be performed. Pericardiocentesis should be reserved for large, hemodynamically compromising pericardial effusions or when fluid is needed for diagnostic purposes.
c. If the etiology is uncertain, pericardial fluid should be sent for a hematocrit and a white blood cell count with differential, glucose, protein, cytologic, and microbiologic analyses (e.g., culture for various organisms and AFB staining). If there is a clinical suspicion of purulent pericarditis, pericardiocentesis should be performed promptly and the fluid sent for culture. If the pericardial fluid is serosanguineous or grossly bloody, the clinician should send it for cytologic examination, culture, and AFB staining.
3. Surgical therapy
a. Subxiphoid pericardiostomy is usually performed for neoplastic pericarditis with rapidly recurrent pericardial effusions. Sclerotherapy with tetracycline has been performed in severe cases of neoplastic pericarditis; however, the procedure is painful and is associated with arrhythmias and risk for constrictive pericarditis.
b. Pericardiectomy is reserved for severe recurrent pericarditis. More commonly it is employed in the management of constrictive pericarditis, as discussed later in this chapter.
F. Follow-up
1. Most patients with idiopathic or viral pericarditis should receive 1-month follow-up to ensure that their symptoms have resolved and that no evidence of constrictive pericarditis exists.
2. Patients with pericardial effusions should have serial echocardiograms to assess for recurrence or an increase in the size of the effusion.
G. Prevention of postpericardiotomy syndrome (PPS).
In a recent multicenter, double-blind, randomized trial, colchicine was found to be useful in preventing PPS and its related complication; however, this approach has not been widely used because of the relatively low incidence of clinically significant PPS.
III. CONSTRICTIVE PERICARDITIS
results from a fibrous thickening of the pericardium secondary to chronic inflammation from a variety of injuries. Essentially, the heart is encased by the rigid pericardium, leading to a decrease in diastolic filling, an increase in intracardiac pressures, and a dissociation of intracardiac pressure from intrathoracic pressure. The hallmark of pericardial constriction is the equalization of end-diastolic pressures in all four cardiac chambers. The elevated cardiac pressures and diminished diastolic filling lead to increased venous pressure, both pulmonary and systemic, and thus to progressive signs and symptoms of right and left heart failure. Although constrictive pericarditis is a relatively uncommon cause of heart failure, recognition of this entity is important, as its prevalence appears to be increasing and the diagnosis is often missed.
A. Causes of constrictive pericarditis.
The
factors involved in the development of constrictive pericarditis are varied and are similar to those of acute pericarditis (
Table 40.3). However, there is
a common pathophysiologic pathway leading to
chronic inflammation and pericardial fibrosis. Neoplastic disease is an exception because tumor infiltration of the pericardium is often responsible for constriction. The causes of constrictive pericarditis in the decreasing order of frequency are idiopathic factors, radiation therapy, postsurgical therapy, and infectious disease. This represents a significant change from a century ago when infectious disease, specifically tuberculosis, predominated.
1. Since the advent of effective antitubercular medications, the number of cases attributable to tuberculosis has dropped precipitously in the United States. However, tuberculosis remains the primary cause of constrictive pericarditis in most developing regions of the world.
2. Similarly, bacterial infections of the chest continue to represent a large number of cases on a global scale, but these have largely disappeared in the United States following the introduction of antibiotics and improved drainage procedures.
3. Most “
idiopathic” cases are
likely infectious in nature, due to viral infections caused by viruses such as
coxsackievirus and echovirus; however, a clear etiologic
link is rarely established. Less common infectious agents include fungal and parasitic organisms.
4. Constrictive pericarditis is a late complication of radiation therapy, generally occurring many years after the administration of radiation. Risk factors for development of constrictive pericarditis include duration of therapy, total amount of radiation administered, and volume of the heart in the radiation field. In contrast to other causes of constrictive pericarditis, where the myocardium is typically normal in structure and function, there may be associated radiation damage to the myocardium.
5. Constrictive pericarditis is a well-documented late complication of cardiac surgery, including coronary artery bypass grafting and valvular surgery. Risk factors for development of postoperative constrictive pericarditis include intraoperative hemorrhage into the pericardium, postoperative pericarditis, and the occurrence of postpericardiotomy syndrome.
6. End-stage renal disease, neoplastic disease (primarily breast, lung, and lymphoma), and connective tissue disease are less common causes that must be considered in the initial differential.