1. General issues
Medical history: previous problems with anesthesia including allergies and current medications; presence of conditions that might affect surgical risk (chronic lung disease, renal disease, liver disease, arrhythmias, hematological disorders, diffuse vascular disease, diabetes, early dementia etc.)
Examination: signs of other important medical conditions (see above)
Biochemistry and general tests: full blood examination, renal and liver biochemistry, group and cross match of blood, urinalysis, chest X-ray, electrocardiogram, and, in selected patients pulmonary function tests and arterial blood gases
2. Specific issues
Recent use of anticoagulants
Recent use of antiplatelet agents
Current control of heart failure symptoms and signs
Optimization of cardiac failure control with medications
Absence of any currently active infection
3. Cardiological issues
Availability and review of all appropriate imaging modalities
Careful assessment of risk/benefit ratio for the decision to operate
Need to modify medications before surgery (temporarily stop clopidogrel or aspirin or warfarin)
4. Surgical issues
Quality of leg veins as conduits
Suitability of radial arteries as conduits
If re-do surgery, suitable information of previous operation
Planning of correct approach for a given patient (off-pump vs. on-pump, number of grafts, type of valve surgery, cardioplegic strategy, etc.)
When the patient comes to surgery from the CCU, ICU or ED, by definition, there must have been a sudden change, which requires prompt surgical intervention. Such change may stem from unstable coronary ischemia, coronary ischemia with aggravation of heart failure, worsening of valve function, decompensation of cardiac function, endocarditis, valve rupture and so on. The treatment of such patients must, of course, include: (1) Stabilization of the physiological state in order to ensure patient safety; (2) Simultaneous management of the exacerbating condition and (3) Carefully timed surgical intervention.
No randomized controlled trials exist to specify a particular pathway of management for all conditions. Each must be dealt with using the specific knowledge, which applies to it. Thus, a given patient may just require antibiotics and optimization of cardiac failure medications, while another will require intubation, mechanical ventilation, inotropic support, intra-aortic balloon counterpulsation, continuous hemofiltration and even extra-corporeal membrane oxygenation or the use of a ventricular assist device.
In fact, the principles which apply to this period are the same as those that apply to post-operative care and will be described in detail later in this chapter.
The Immediate Post-operative Period
The most important single predictor of a safe post-operative period is the successful execution of a well-planned and correctly applied operation.
However, even when this is done, several patients with heart failure require very careful and skilled medical management. If the operation is ill-conceived or ill-executed or the patient is extremely unwell ever before surgery, the patient will experience life-threatening problems, which, sometimes require extraordinary feats of technology and great team work to achieve survival and recovery.
Monitoring and Inotropic Drugs
A patient with heart failure undergoing surgery with cardiopulmonary bypass will inevitably experience a decrease in myocardial contractility, which, combined with the pre-operative state of heart failure, often mandates the use of intropic drugs. This decrease in contractility is mediated by complex mechanisms [1, 2] and typically results in a progressive post-operative decrease in contractility with a nadir in contractile function between 8 and 24 h after cardioplegia, depending on the duration of cardiopulmonary bypass (CPB).
Accordingly, almost all of these patients require inotropic support. Although, no randomized controlled trials exist, in the opinion of the authors, it is generally best to monitor cardiac output in cardiac surgery patients and it is vital to do so in those who have surgery in the context of impaired pre-operative myocardial function (heart failure patients). Monitoring of cardiac output allows physicians to either prevent or rapidly detect and treat a low cardiac output state. Such treatment initially requires a judicious combination of three components: (1) Inotropic drugs; (2) Fluid resuscitation; (3) Control of heart rhythm and rate (pacing).
There are two major classes of inotropic drugs that can be used in patients with cardiac surgery in the setting of a postoperative heart failure and/or a low cardiac output state (LCOS): catecholamines and phosphodiesterase III inhibitors (PDEIs) [3–13] (Table 18.2). More recently, they have been joined by a new class of agents called calcium sensitizers [14]. However, experience with these new inotropic agents in cardiac surgery is limited [15]. These agents have different properties (Table 18.2) and have never been compared in suitably powered randomized controlled trials of heart failure patients having cardiac surgery to test whether the use of one or the other results in better clinical (instead of physiological) outcomes. Accordingly, they are typically used according to local (institution) and individual (physician) preferences. In addition, there is no consensus definition of what constitutes the goal of inotropic therapy. In general, however, inotropic agents are administered to deal with a low cardiac output syndrome (LCOS) in order to either prevent its occurrence or return cardiac output (CO) to adequate levels to ensure sufficient oxygen delivery to tissues.
Table 18.2
Inotropic drugs
Agent | Significant features |
|---|---|
Epinephrine | Increases CI with biphasic effect on SVRI. Produces rise in serum lactate |
Dopamine | Increased SVRI at doses above 5.0 μg/kg/min. Less clinical efficacy than dobutamine, dopexamine, amrinone or enoximone. Increased incidence of adverse cardiac events than dopexamine |
Dobutamine | Better efficacy than dopamine and epinephrine. Decreases SVRI. Tachycardia and tachyarrythmia (esp. AF) more common |
Dopexamine | Greater tachycardia than dobutamine. More efficacious and less adverse events than dopamine |
Amrinone | Improved weaning from CPB. Improves CI and decreases SVRI with minimal effects on HR. Reports of thrombocytopenia associated with use |
Enoximone | Significant increase in CI without tachycardia. Decreases SVRI. As effective as dobutamine |
Milrinone | Significant increase in CI without tachycardia. Decreases SVRI. As effective as dobutamine, but less AF. Lusitropic. Improves graft flow. As effective as 20 ppm of inhaled nitric oxide in pulmonary hypertension |
No consensus definition of what a LCOS currently exists. However, it would be reasonable to define it as any low cardiac output state (cardiac index of <2.4 L min−1 m−2 is used as a criteria in some studies) with clinical and laboratory evidence of inadequate peripheral perfusion (e.g. a persistently elevated lactate, cool vasoconstricted hands and feet, a urine output persistently <0.5 ml h−1 for more than 1 h, evidence of ischemic hepatitis).
Such LCOS can continue for several hours to days, despite optimisation of volume status, temporary pacing, exclusion of mechanical factors (e.g. cardiac tamponade or pneumothorax) and mechanical assistance with intra-aortic balloon counter pulsation (IABP). Causes for this LCOS are multifactorial but include myocardial ischemia during cross clamping, reperfusion injury, cardioplegia-induced myocardial dysfunction, activation of inflammatory and coagulation cascades and un-reversed pre-existing cardiac disease. LCOS can result in reduced oxygen delivery to vital organs. Such end-organ ischemia will lead to multiorgan failure. Initial organ dysfunction and multiple organ failure are among the main causes of prolonged hospital stay after cardiac surgery and they increase resource use and healthcare costs as well as morbidity and mortality. Optimisation of cardiac output and oxygen delivery may, therefore, decrease morbidity and reduce length of stay and remains the cornerstone of hemodynamic management.
It must be emphasized here that a particular value for the cardiac index must always be interpreted within the clinical context. A cardiac index of 1.8 L/m2/min may be perfectly adequate in a patient with a normal lactate, a urine output of 1 ml/kg/h and a core temperature of 35.5 °C immediately after transfer to the ICU. In such a patient, hypothermia is likely mostly responsible for decreased metabolic demand and the low value of the cardiac index. Nonetheless, in all patients the cause for the low cardiac index must be diligently sought and dealt with. In particular, one must always be vigilant about the possibility of cardiac tamponade or pneumothorax or other mechanical factors which impede cardiac output. Their diagnosis requires a high index of suspicion, regular patient examination and review and the prompt use of chest X-rays and echocardiography.
The use of inotropic drugs is often insufficient to restore cardiac output if the patient’s heart rate (HR) is not optimized. Patients with cardiac failure who have been on beta-blockers until the time of surgery will often be bradycardic post-operatively and will have a LCOS because of such bradycardia in the setting of a low stroke volume, unless their hear rate is optimized. This is because CO = stroke volume (SV) × heart rate (HR). In these patients, even with inotropic drugs and optimal fluid therapy, SV can only be partially increased, therefore optimal HR is vital. This optimization is best achieved by epicardial pacing of the atria in order to maintain atrial contractility. If the patient is chronic atrial fibrillation, ventricular pacing is necessary to maintain an adequate rate. The importance of pacing in the post-operative period in patients with pre-operative heart failure cannot be overemphasized. Pacing not only provides the ability to optimize heart rate but also allows the prompt and safe treatment of tachyarrhythmias like atrial fibrillation, which so commonly occurs after cardiac surgery in these patients.
In particular, the availability of pacing makes the use of intravenous amiodarone extremely safe [16].
Post-operative Fluid Therapy
Fluid therapy is a source of incessant controversy in the post-cardiac surgery period because of the lack of randomized controlled trials. Such controversy also arises from the need to individualize care and to change such individualized care dynamically and frequently as the patient’s hemodynamic state changes over hours and sometimes days following surgery [17]. However, some comments are in order.
First, there is insufficient evidence that a particular kind of fluid is better than another. A recent large randomized controlled trial in critically ill patients has shown no difference in overall outcome between patients treated with saline compared to patients treated with albumin [18]. Accordingly, both colloids or crystalloids are theoretically acceptable choices. However, there is widespread concern about inducing fluid overload states and about the capillary leak state that most cardiac surgery patients experience after CPB. Therefore, most of the fluid literature for cardiac surgery patients appears to preferentially report the use of colloidal fluid preparations. These typically include either natural colloids such as albumin or artificial colloid solutions such as starch and gelatin preparations [19].
Second, there is always controversy about “adequacy of filling” in these patients (i.e. what the optimal left or right ventricular end diastolic volume might be in a given patient at a given time). This is also due to the lack of randomized controlled trials and the dynamic nature of all measurements and physiological states. Further uncertainty is added by the fact that myocardial filling cannot be reliably assessed by currently applied forms of hemodynamic monitoring [20]. In particular standard pulmonary artery technology measures pressures, not volumes. The relationship between pulmonary artery catheter derived pressures and end diastolic filling volumes is highly unpredictable [20]. New technologies are being applied beyond the traditional pulmonary artery catheter such as pulse contour cardiac output analysis by transpulmonary thermodilution [21] and transesophageal echocardiography [22]. Their usefulness in the ICU and ability to deliver superior outcomes remain unknown. In the opinion of the author, echocardiography and thermodilution technology are complementary and should be used in unison and together with clinical and laboratory assessment of the patient’s condition to help guide hemodynamic management. Importantly, however, and irrespective of echocardiographic findings if a fluid challenge (250 ml of IV colloid bolus over 10–15 min in an adult patient) fails to increase cardiac output (CO) by >15 %. Then the patient is in the flat portion of the Starling curve. Logically, in such patients, further administration of fluid will provide no benefit and will potentially induce chamber dilatation. Such dilatation and the increased intracavitary pressures that go with it will likely decreased endocardial perfusion (thus worsening cardiac output), increase the probability of pulmonary edema, myocardial edema and vital organ edema. Thus, such unnecessary fluid therapy should be avoided. In mechanically ventilated patients who do not have spontaneous respiratory effort he measurement of pulse pressure variation (the percentage change in mean arterial pressure induced by a ventilator breath) may help predict which patients will respond to a fluid challenge with a >15 % increase in cardiac output.
Particularly seductive and misleading is the appearance of an “underfilled” left ventricle on echocardiography in the setting of right ventricular dysfunction with an already enlarged right ventricle and a high right atrial pressure (>15 mmHg). Further filling will predictably result in the following: no change in left ventricular filling, septal movement into the left cavity with decreased left ventricular compliance, further right ventricular dilatation, right ventricular endocardial ischemia, increased back pressure in the liver, liver cell ischemia and no change or even a decrease in cardiac output. In this setting, the correct treatment is the use of inotropic support, pulmonary vasodilatation with nitric oxide and, if these measures appear insufficient, institution of mechanical support (IABP or extracorporeal membrane oxygenation or ventricular assist device).
Vasopressor Drugs
Hypotension is relatively common after cardiac surgery. While a degree of hypotension may be clinically unimportant, a low mean arterial blood pressure (MAP <65–70 mmHg) may be undesirable in some patients (carotid artery disease, renovascular disease) and, in general, should be prevented or treated. A very low blood pressure (MAP <60 mmHg or diastolic BP <40 mmHg) frequently causes renal dysfunction, threatens the adequacy of liver perfusion and may induce inadequate coronary blood flow with sub-endocardial ischemia, especially in the setting of high pulmonary artery occlusion pressures (PAOP). In particular, if the diastolic BP is 40 mmHg and the PAOP (a surrogate for left ventricular end diastolic pressure) is 25 mmHg, coronary perfusion pressure for the left ventricle will be 15 mmHg. This value is <25 % of normal and is likely reduce coronary blood flow significantly, especially in patients with a hypertrophic ventricle [23]. The same is true for liver blood flow in the setting of a LCOS, right ventricular dysfunction and an elevated central venous pressure [24].
The pathogenesis of hypotension after cardiac surgery in heart failure patients is typically complex and may involve a variety of factors either alone or in combination (Table 18.3). All should be considered and either excluded or identified by means of prompt clinical, radiological, hematological and hemodyamic assessment.
Table 18.3
Factors potentially responsible for hypotension after cardiac surgery in heart failure patients
Low cardiac output syndrome |
Relative or absolute hypovolemia |
Bleeding |
Tamponade |
Pneumothorax |
Inflammatory response to CPB |
Fever |
Drugs used for sedation |
Use of inodilators (PDEIs) |
Residual effect of pre-operative ACE inhibitors |
Patient-ventilator dyssynchrony |
If the patient has an adequate or even high cardiac output and all other factors have been excluded, then one is likely dealing with an “inflammatory vasodilatory state” or so-called “post-CPB vasoplegia” [25]. In these patients, if the blood pressure is too low, the use of vasopressor drugs becomes necessary.
There are several vasopressor agents, which can be used for this purpose (norepineprhine, phenyephrine, vasopressin). The agent for which there is greater clinical experience, however, is norepinephrine (noradrenaline). Despite theoretical concerns over its adverse effects on renal and mesenteric blood flow, most of the available data in fact indicate this agent is efficacious and safe under these circumstances [26], particularly from the renal functional point of view (Fig. 18.1).
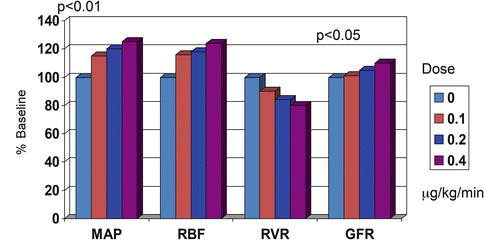

Fig. 18.1
Comparison of changes in renal function in dogs with the administration of increasing doses of norpineprhine (noradrenaline) (MAP mean arterial pressure, RBF renal blood flow, RVR renal vascular resistance, GFR glomerular filtration rate). Norepinephrine infusion up to 0.4 mcg/kg/min increases MAP, RBF and GFR and decreases RVR
In some patients with pre-operative sepsis (e.g. endocarditis), this vasodilatory state can be dramatic and may require the addition of vasopressin [27]. Assuming that external mechanical factors have been corrected, the approach to the hemodynamic management of these patients can be summarized in a flow chart (Fig. 18.2).
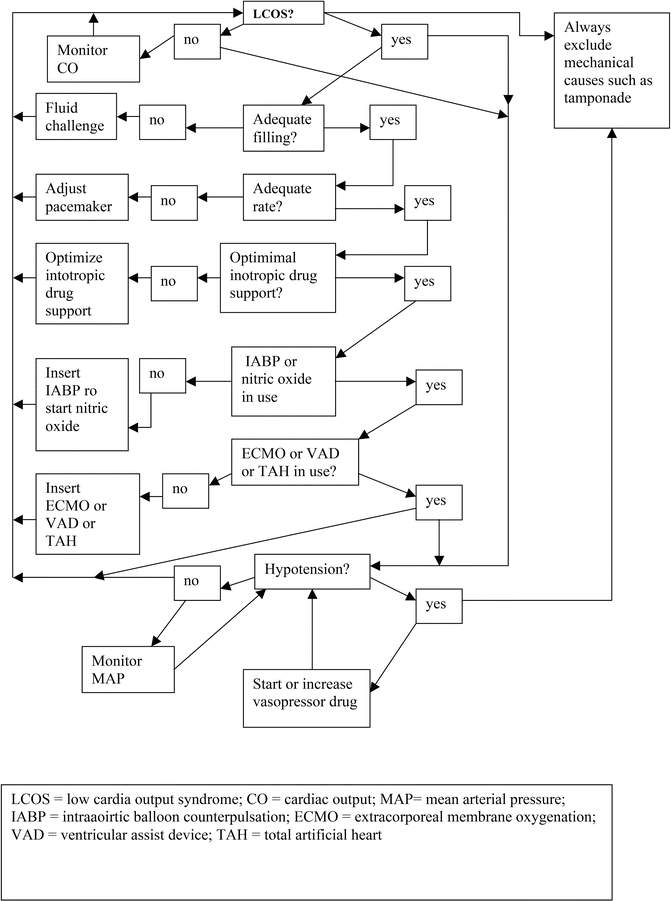

Fig. 18.2
Flow diagram for hemodynamic management
Mechanical Support
Some patients with heart failure have severe cardiac functional impairment after surgery. Despite the use of inotropic agents, optimization of fluid therapy, optimization of pacing and the addition of vasopressor drugs to restore adequate vital organ perfusion pressure, and nitric oxide to decrease pulmonary vascular resistance, some of these patients continue to have either a LCOS or hypotension of both. In these patients, mechanical support should be rapidly considered and implemented.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


