Fig. 13.1
Severe ischemia in the distribution of the left anterior descending coronary artery seen by MR perfusion (top row) and SPECT imaging (bottom row). A single frame from the first-pass MR perfusion series demonstrates severe ischemia of the anteroseptum from base to apex in the three-chamber view (top left) and of the septal and anterior walls in the mid-chamber short axis view (top right). On SPECT imaging, a similar distribution of ischemia is seen on the vertical long axis (bottom left) and mid-chamber short axis view (bottom right)
The most common way to measure perfusion with CMR is via the “first pass” technique. A bolus of contrast is injected in a peripheral vein, and contrast enhancement is observed as the bolus passes through the myocardium. Mathematical analysis of the shape of the time-signal intensity curve measured in the myocardium is used to quantify the tissue perfusion in ml/g-min. There are other techniques for measuring perfusion with MRI, but they have notable drawbacks. Arterial spin labeling (ASL) [10] is a promising technique that uses no exogenous contrast, but has less contrast to noise ratio (CNR) than traditional first pass methods. Blood Oxygen Level Dependent (BOLD) imaging [11] also uses no exogenous contrast, but its interpretation for quantitative perfusion can be complicated because it also depends on factors such as oxygen extraction fraction. Because first pass with a contrast bolus makes up the overwhelming majority of cardiac perfusion scans, it will be the focus of this chapter.
Pulse Sequences
Constraints and Requirements
The choice of which pulse sequence to use is a key determinant of image contrast, spatial and temporal resolution, coverage, and degree of artifacts. These characteristics are usually at odds with one another – for example gains in contrast may come at the expense resolution or coverage – and selecting the optimal sequence requires weighing these competing gains and losses against each other. Consequently, when selecting a perfusion sequence, it is critical to consider the basic requirements for cardiac perfusion imaging. These are:
1.
Strong T1 contrast. The contrast agent used in MRI perfusion significantly alters the T1 of the myocardium during first pass, therefore T1 sensitive image contrast is desirable.
2.
Coverage of relevant myocardium. Usually this includes at minimum one short axis slice each through the base, middle, and apex of the myocardium of the left ventricle (LV).
3.
Spatial Resolution. At minimum, most sequences must be able to distinguish between subendocardial and transmural ischemia.
4.
Temporal Resolution. To adequately sample flow at the myocardial level, images must be acquired every one to two heartbeats. For quantitative perfusion, there is the additional requirement of being able to adequately sample the input function within the blood pool of the LV. Because contrast passes much quicker through the LV than the myocardium, this usually requires sampling every heartbeat.
5.
Lack of Artifacts. In particular, the dark rim artifact is a specific property of MRI perfusion images that can mimic perfusion defects resulting in potential for misinterpretation and should be minimized.
In meeting these requirements, perfusion imaging has a critical constraint that is absent in much of CMR: because characterizing the bolus passage requires acquiring a full image every one to two heart beats, segmented acquisition (as is used in Cine and Late Gadolinium Enhanced imaging) is limited or impossible. In order to achieve full heart coverage, typically three short axis images must be acquired within the time course of a single heartbeat. This limits perfusion imaging to very time-efficient pulse sequences that reduce the time to acquire an image to ~100 ms. The image acquisition time is determined by the length of the cardiac cycle which is often shortened by vasodilator stress.
Preparation Pulses
At the most basic level, a T1-weighted image is acquired by perturbing the longitudinal magnetization (MZ) away from equilibrium through the application of an RF pulse at the beginning of the pulse sequence and then acquiring images before the magnetization has a chance to return to normal. As a general rule, blood, fat, and myocardium with short T1 will return to its unperturbed state faster, appearing bright in a T1-weighted images. There are two approaches to perturb MZ in cardiac perfusion imaging: with a 180° (i.e. an inversion) RF pulse in inversion recovery (IR) exams and a 90° RF pulse which completely eliminates or “saturates” MRI signal in a so-called saturation recovery (SR) exam. The SR and IR pulses are often referred to as magnetization preparation pulses and are separate from the RF pulses played during the image acquisition or image “readout”, both of which must be considered in the optimization of a cardiac perfusion exam.
Historically, IR was used for CMR perfusion. Because IR is a 180° pulse, it is has the potential for the most dynamic range and hence most contrast (Fig. 13.2). However, MZ after the pulse is dependent on MZ immediately prior to the pulse (it will be the same magnitude but point in the opposite direction). This means that the length of the previous TR will affect the magnitude of Mz after inversion pulse. In a gated cardiac scan, the TR is roughly equal to the RR interval. This makes scans with IR preparation very sensitive to changes in heart rate and arrhythmias because the RR and TR change throughout the scan. The other disadvantage of IR is that it requires a longer readout because it takes longer for the magnetization to recover. Practically, this means that more time is required per slice, so fewer slices can be acquired, and there is a decrease in spatial coverage.
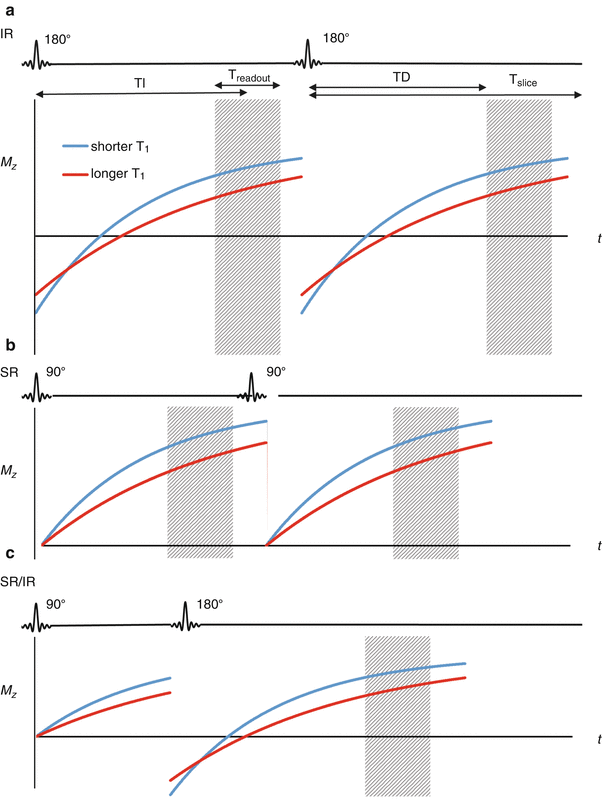

Fig. 13.2
(a) Saturation recovery (SR), (b) inversion recovery (IR), and (c) hybrid SR-IR preparations for myocardial imaging. IR has a greater signal range but is slower and is susceptible to variations in heart rate (note that Mz after the inversion pulse is dependent on Tslice/T1). For the hybrid SR-IR, the initial SR preparation removes any heart rate variability, and the following IR pulse increases signal range over an SR only prep. TD trigger delay, TI inversion time (time from preparation to center of k-space), T readout total time for acquisition of all k-space data for a single slice, T slice total time required for acquiring a single slice. Note that TI is TD plus half Treadout
Currently, the majority of CMR perfusion scans are acquired using SR preparation. While there is less dynamic range and contrast because it is only a 90° pulse (see Fig. 13.2), SR does not have the other drawbacks of IR. Critically, SR pulses will always set Mz to zero regardless of the prior Mz, so the signal has no heart rate dependence. Additionally, because the readout is faster, more slices can be acquired allowing for greater spatial coverage.
Other pulse preparations are currently being developed. Some, like magnetization driven steady state, offer better linearity at the expense of CNR, which may be useful for some quantification applications. Others are a hybrid of SR and IR preparations. For example, an SR prep followed by an IR prep [12] will exhibit some characteristics of each. There will be no heart rate dependence, and CNR will be improved over a simple SR prep, but readout would take even longer and coverage would decrease. This could be used in cases where good CNR is important but coverage is not (e.g. a diffuse process like systemic sclerosis).
For a 90° SR preparation, there are a few different choices for how to implement the RF pulse. A rectangular pulse is the simplest, however in the presence of any B1 field inhomogeneity (common in cardiac imaging) a rectangular pulse will result in incomplete saturation. Other RF pulses have been designed that have improved performance in the presence of B1 inhomogeneity. Two important ones are adiabatic pulses [13] and rectangular pulse trains [14]. Both of these show markedly improved saturation (Fig. 13.3) [6, 14]. However their drawbacks are longer pulse durations and higher SAR. The longer pulse duration is minor compared the image acquisition time (~8 ms compared to ~150–200 ms), but additional SAR can be problematic with large coverage at higher field strengths (3 T).
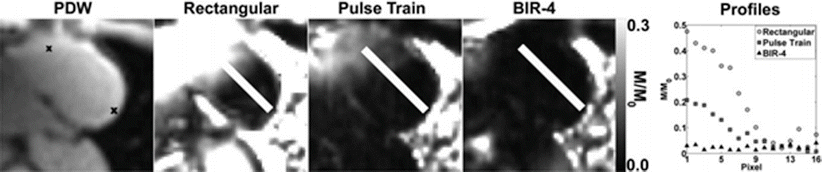

Fig. 13.3
Short axis images demonstrating saturation inhomogeneity with different types of SR preparation pulses. “x” marks on the PDW image mark the end points of the measured signal intensity. For complete homogeneous saturation, the signal should be zero across the entire line. The pulse train shows improved homogeneity over a simple rectangular pulse, and the BIR-4 adiabatic pulse is even more homogeneous (Reproduced from Kim et al. [14], with permission of John Wiley & Sons)
Image Readout: Snapshot FLASH, SSFP, GRE-EPI
Each type of magnetization preparation pulse can be combined with different types of image readout. The most common types are ultra fast gradient echo (e.g. TurboFLASH, fast GRE, and Turbo Field Echo) [15], gradient echo with echo planar readout (GRE-EPI) [16], or steady state free precession (SSFP – termed TrueFISP, FIESTA, balanced FFE by various vendors) [17], and despite much debate, there is no clear consensus for which sequence is best for CMR perfusion and is usually determined based on the preference of the physician.
Fast Low Angle Shot (FLASH) was one of the first available rapid imaging sequences. With FLASH, each line in k-space is preceded by a low flip angle excitation and then any transverse magnetization at the end of the readout is spoiled before moving to the next line (Fig. 13.4). Since each line has its own excitation, the imaging time for the readout (not including preparation) will be Treadout = TR * Nphase. E.g. for a 128 × 80 matrix at TR = 2 ms, the readout time would be 160 ms. (Note: TR here refers to time between excitations during the readout, it does not refer to the time between successive image acquisitions.) TurboFLASH is a variation on FLASH with very short TR and low flip angle. As a consequence, TurboFLASH is predominantly proton density weighted instead of having the typical T1 FLASH weighting. However, with IR or SR prep a TurboFLASH sequence will have T1 weighting.
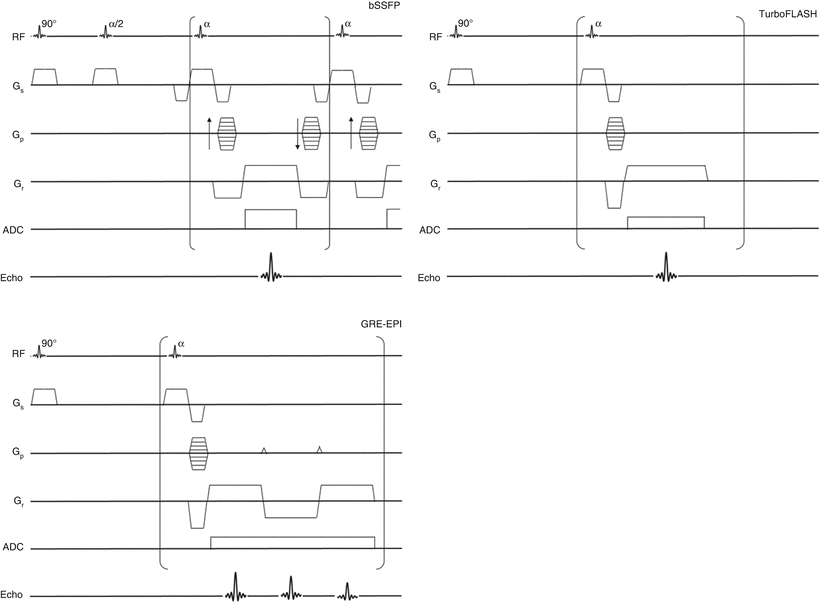

Fig. 13.4
Pulse sequence diagrams for bSSFP, FLASH, and GRE-EPI readouts
GRE-EPI is similar to FLASH, except that instead of reading out one line of k-space per acquisition, multiple lines are read out. This means that fewer excitations are needed during readout, which makes GRE-EPI more efficient and hence faster than FLASH. The number of lines read per excitation is referred to as the echo train length (ETL). For GRE-EPI, the readout time will instead be Treadout = TR * Nphase/ETL. For example a 128 × 80 matrix at TR = 6 ms with an ETL of 4, the readout time would be 80*6/4 = 120 ms. The imaging readout time is particularly important in CMR because motion artifacts are reduced with shorter readout times. This makes GRE-EPI less sensitive to motion than FLASH. Additionally, the faster readout time reduces the overall imaging time per slice, which means more slices can be acquired during each heartbeat. One drawback of GRE-EPI, however, is that magnetic field imperfections accumulate through the extended readout. The accumulation of error manifests itself as additional phase, which can mimic the intended phase used to localize signal within an image. The result is that EPI images can exhibit phase related “ghosting” artifacts.
Steady State Free Precession (SSFP) images are a variant of FLASH. Unlike in FLASH, in SSFP, transverse magnetization (Mx–y) is not eliminated before the next excitation. Rather than being destroyed (i.e. spoiled) by the application of phase-modulated RF pulses or large field gradients, the residual transverse magnetization is refocused and combined with newly excited transverse excitation to dramatically increase the magnetization used in the formation of an image (Fig. 13.4). This gives SSFP greater SNR than either FLASH or GRE-EPI. Also, because initial magnetization depends on both the longitudinal magnetization and the refocused transverse magnetization, SSFP will have some T2 as well as T1 contrast. This makes TE particularly important for SSFP readouts. Like FLASH, SSFP requires one excitation per readout line, and has comparable readout times. Some studies have found more dark rim artifacts with SSFP [18, 19]. Magnetic field inhomogeneity, which can be problematic in the chest where the lungs impart large local magnetic susceptibility changes, is also a problem in SSFP readouts, which has limited SSFP adoption at 3 T. It has also been reported that the excitations in SSFP can interfere with accurate ECG gating [17]. Still, in spite of these drawbacks, the gain in SNR has seen SSFP become increasingly popular in CMR perfusion.
Despite multiple studies comparing the above methods, there is no clear-cut consensus as to which is the best method, and each one offers advantages and disadvantages. In a review article, Kellman and Arai compared SSFP, FLASH, and GRE-EPI using state-of-the-art implementations in 2007 [6]. They found that GRE-EPI had the fastest acquisition, but that SSFP has 40 % higher CNR.
Acquiring Multiple Slices
Typically CMR perfusion scans require multiple slices for adequate coverage of the LV myocardium supplied by the coronary arteries. The most common method for acquiring multiple slices is to use multiple SR preps. The first slice is SR prepped and read out, then the second slice is SR prepped and read out, etc. until all the slices are completed. Another approach is to use a single SR prep and then read out multiple slices. This is faster than using multiple SR preps, so greater spatial coverage is possible. However, the time between the SR prep and readout will be different for each slice. This leads to a very important disadvantage: each slice will have a different TI, so each slice will have different CNR. A third possibility is to use a single SR prep but interleave the slice readouts. This keeps the same efficiency gains but equalizes the TIs, so there is no CNR variation between slices. However, the readout per slice is longer, which increases susceptibility to motion artifacts.
Acceleration Techniques
Due to the need for very fast image acquisition, cardiac perfusion sequences are almost always run with some sort of acceleration technique. Acceleration techniques include parallel imaging (e.g. SMASH [20], SENSE [21], and GRAPPA [22]), k-t Blast/k-t SENSE [23], and HYPR [24, 25]. Much research has been focused on acceleration techniques in recent years, and a multitude of techniques have been developed and compared [26, 27]. In all of these techniques, image acquisition time is reduced by intentionally sampling only a subset of the data needed to create an MRI images. The uncollected or “missing” MRI data are mathematically synthesized using complimentary information collected from different receiver coils. In other words, most acceleration techniques use the spatial location of the acquired signal which is inherent in the receiver coil configuration to reduce the amount of imaging data that must be acquired for artifact free images. Since receiver coil information is acquired simultaneously, i.e. in parallel, the general approach to accelerate is referred to as “parallel imaging”. However, a major tradeoff in all cases is that faster acquisition results in lower SNR. A review of parallel imaging basics can be found by Deshmane et al. [28].
Sensitivity encoding (SENSE) and generalized autocalibrating partially parallel acquisition (GRAPPA) are two widely available methods of parallel imaging, and both have major advantages in that they rely on relatively simple theoretical underpinnings and make very few assumptions about the nature of the underlying images. With parallel imaging, reconstruction additionally incorporates information from multiple independent receiving coils. In essence, spatial information that would otherwise be obtained by spatial encoding via gradients is instead obtained by information in independent coils in the receiver coil array. With SENSE, coil sensitivity profiles are used to unwrap the aliased images in image space. With GRAPPA, the unsampled lines in k-space are calculated by combining information from neighboring lines in multiple coils, and the filled-in k-space is then reconstructed as usual. This eliminates the ghosting artifacts that would normally be seen by undersampling, and the more receiving channels that are used, the more k-space can be undersampled and acquisition speed increased.
Other techniques achieve even greater acceleration by incorporating temporal information. For most rapidly acquired image series of the heart, much of the image remains unchanged between images, and data is correlated in time. k-t BLAST and k-t SENSE are two well-known techniques that take advantage of this correlation by acquiring a training data set (acquired at low resolution and un-aliased) that is used to inform the reconstruction of the sparsely sampled and rapidly acquired data. k-t BLAST does not incorporate coil channel information in its reconstruction and can be used with single channel coils whereas k-t SENSE incorporates coil channel information as well. Compared to standard parallel imaging techniques, k-t BLAST and k-t SENSE are capable of faster imaging, but at the cost of increased noise and more assumptions in the model (e.g. that motion during the training data is representative of motion in the rest of the data).
Motion Correction
While many cardiac MRI scans rely on breath holds to ensure that there is minimal movement of the heart during the scan, the longer acquisition times of a cardiac perfusion scan (typically 45 s–1.5 min) can make breath holds impractical. As a result, there is usually considerable cardiac motion over the course of a perfusion scan. This is problematic when analyzing perfusion images. Generating signal intensity time curves requires segmenting along the epi- and endocardial borders, but this is an extremely time intensive process to do frame by frame, and automatic segmentation often does not trace the borders well. A more efficient process is to first register all of the images together, draw the contours on a single image, and then propagate the contours throughout the series and make (relatively minor) adjustments as needed. As such, most cardiac perfusion scans will include some form of motion correction for image registration.
There are a multitude of motion corrections algorithms that have been proposed. Motion can be corrected prospectively using navigator pulses that track the motion of the diaphragm [29]. Motion can also be corrected retrospectively using a variety of methods [30–33]. In practice, many vendors will have some form of inline motion correction included in their cardiac sequences. A comprehensive review of cardiac motion correction can be found in the review by Scott et al. [34].
Artifacts
There are several artifacts seen in CMR perfusion imaging, and the most important one is the dark rim artifact (DRA) (Fig. 13.5). The DRA manifests as a dark rim that is sometimes seen in the subendocardial border of the ventricle. This ring can easily be mistaken for hypoperfusion and cause incorrect diagnoses, which is why DRA is regarded as the most concerning artifact in CMR. Much research has gone into determining the cause of the DRA, and some common hypotheses include Gibbs ringing, contrast associated susceptibility changes, motion artifacts, and partial volume effects [35]. However, no theory has been clearly identified as the sole cause of DRA, and its origins remain widely debated.
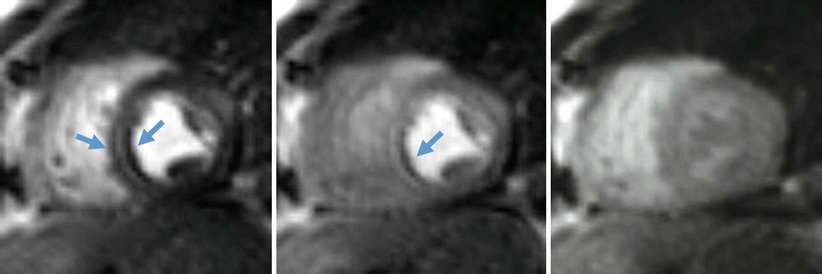

Fig. 13.5
Dark rim artifact (DRA). Note that the artifact is only apparent when there is contrast in the adjacent ventricle and does not persist after the first-pass
1.5 T vs 3 T
While the majority of clinical scanners use 1.5 T magnets, 3 T is becoming increasingly common, and the choice between the two has a significant effect on CMR perfusion. The higher magnetic field of the 3.0 T results in a doubling of the signal-to-noise ratio of images and a 30 % prolongation of T1 values [36]. Importantly, Gadolinium-based contrast agents have less relaxivity at 3 T. However, since T1 values are also higher in the blood and myocardium at 3 T than at 1.5 T there is a net increase in ΔT1 and gain in CNR [37].
While 3 T offers CNR advantages, it has other disadvantages. Artifacts are more prominent at 3 T, though faster imaging and higher bandwidth can mitigate artifacts at the cost of SNR. Critically for SSFP sequences, there is more inhomogeneity at 3 T [38]. There is also greater energy deposition as quantified by the Specific Absorption Rate (SAR) at 3 T, which limits the TR and flip angles that can be used, and ECG signal is noisier at 3 T, which can influence any scan where accurate gating is critical.
Summary
There are a multitude of choices available when creating a CMR perfusion sequence. Preparation can be IR, SR, or a hybrid, and there are multiple ways to implement the preparation. Readouts can be some variation of FLASH, GRE-EPI, or SSFP. There is no single best combination for all applications, and selection of the “best” sequence depends on the specific needs of a particular scan. For example, relative to ischemia, post infarction imaging involves more microvascular than macrovascular obstruction, so contrast washes out more slowly [8]. In this case, some temporal resolution could be sacrificed to gain greater spatial resolution to better delineate the size of injured myocardium. Conversely, for a diffuse process like microvascular dysfunction in Syndrome X, spatial coverage could be neglected in favor of having greater spatial resolution in fewer slices to evaluate subendocardial hypoperfusion.
Image Analysis
For any given image series, there are a multitude of different ways to process the data and provide an assessment of cardiac perfusion. Recent articles have reviewed and compared various methods [8, 9, 39], there is no clear consensus on the best approach. Broadly speaking, perfusion analysis can be categorized as qualitative, semi-quantitative, or absolute quantitative where parametric images present signal intensity in proportion to ml/-g-min of perfusion. Absolute quantification allows a more direct comparison in cross-sectional and longitudinal studies of perfusion changes within individual patients and in patient populations.
Qualitative
The simplest way of analyzing perfusion data is to simply visually inspect myocardial signal changes as the bolus of contrast agent passes, which is what is done in most clinical applications. A physician will cycle through the perfusion series and watch the myocardium as the contrast flushes through. Any areas that remain dark have less perfusion than the surrounding bright tissue. Comparison of stress, rest, and late gadolinium enhanced images allows defects to be attributed to ischemia, infarction, or artifact. Defects seen at stress but not at rest are interpreted as reversible ischemia. Matched defects seen at stress and rest with a corresponding area of late gadolinium enhancement are interpreted as infarction. Matched defects seen at stress and rest without any late gadolinium enhancement are interpreted as artifact. This algorithm improves the diagnostic accuracy over interpretation of the perfusion images alone [40].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


