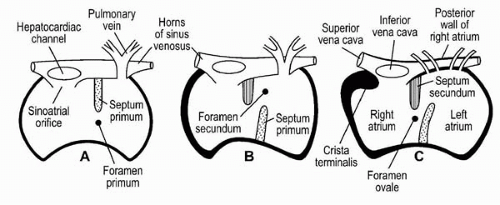
of a thin hypermobile redundant membrane in the area of the fossa ovalis. In practical terms, a septum with a maximal excursion into either the right and/or left atria of > 10 mm is typically used to define the presence of an ASA. ASAs are much less common than PFOs, with an incidence (as assessed by TEE) of approximately 2%, and an associated PFO is found in >50% of these patients.
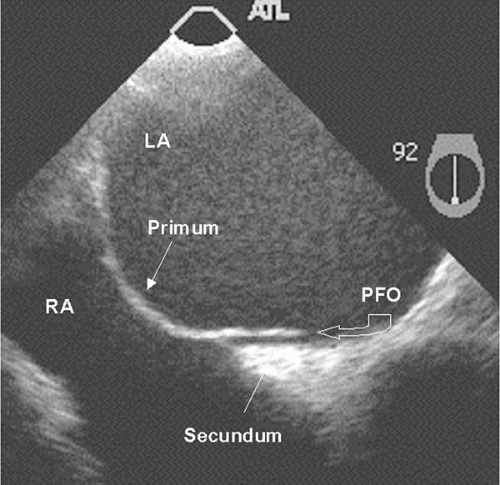 Figure 24.2. TEE images of patent foramen ovale (PFO). This PFO has a tunnel-like appearance. RA, right atrium; LA, left atrium. |
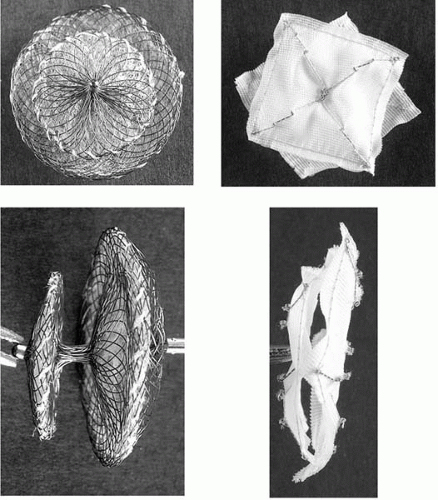 Figure 24.3. Photographs of the Amplatzer PFO occluder (left) and CardioSEAL (right) devices en face and in side profile. |
TABLE 24.1. LIST OF CLINICAL, IMAGING, AND LABORATORY VARIABLES THAT FAVOR THE ROLE OF PARADOXICAL EMBOLISM ACROSS A PFO AS THE ETIOLOGY OF CRYPTOGENIC STROKE | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
and arterial oxygen desaturation that is induced or accentuated by the upright position (13). Several case series of percutaneous PFO closure for patients with POS have been reported, with improvement noted in symptoms and arterial oxygen saturation (to >95%) in the upright posture in most patients (14,15), thus making this a valuable therapeutic option in symptomatic patients. In contrast, persistent desaturation occasionally is encountered in patients with an abrupt elevation in right atrial or pulmonary artery pressures in the setting of right ventricular infarction, acute pulmonary embolism, or acute tricuspid regurgitation (e.g., immediately post-cardiac transplantation). Because many of these events are associated with gradual resolution of elevated right atrial and pulmonary pressures, a conservative approach avoiding percutaneous PFO closure is advised, unless the hypoxia is severe.
TABLE 24.2. SUMMARY OF DESIGN AND CHARACTERISTICS OF CURRENTLY AVAILABLE PFO CLOSURE DEVICES IN THE UNITED STATES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree