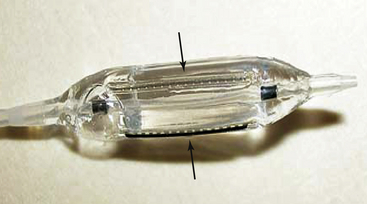
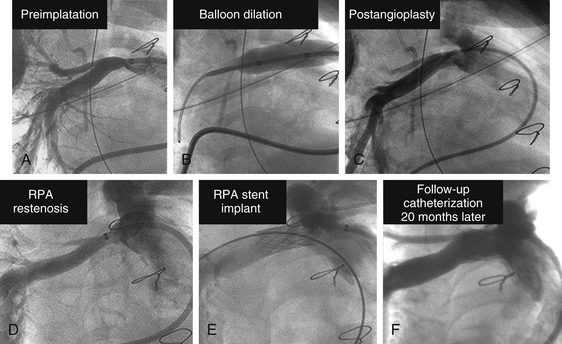
Chapter 23 1. Cardiovascular obstructions in adult congenital heart disease (CHD) often involve branch pulmonary artery stenosis and postsurgical baffles, homografts, and conduits. 2. Stenting various cardiovascular obstructions in adult CHD requires a good understanding of available stents and balloons of all sizes and all of their unique characteristics. 3. Intravascular stenting should accommodate for adult growth potential of the patient. 4. Accurate measurements and basic techniques of angioplasty and stenting of large pulmonary and systemic vessels are essential in the management of adult CHD. Relief of vascular obstruction initially described by Dotter and Judkins1 for peripheral atherosclerotic disease is now routinely applied to a variety of cardiovascular obstructions found in congenital and postsurgical heart disease. Advances in catheters, wires, stents, and therapeutic techniques allow for advanced interventions to relieve these obstructions from the newborn infant to the elderly adult. Although pediatric interventionists manage the majority of these lesions, as CHD patients survive to adulthood and enter into the adult clinics and hospitals, adult interventional cardiologists should become familiar with the diagnosis and management of these lesions as well. Much procedural knowledge, technique development, and refinement in the use of static balloon angioplasty can be traced to the results of the Valvuloplasty and Angioplasty of Congenital Heart Disease Registry (VACA).2 Although there are many manufacturers of balloons who use different materials, sizes, and lengths, all balloons impart a certain advantage or disadvantage for specific lesions. It is imperative for an active catheterization lab to carry and become familiar with a wide array of inventoried balloons. An ideal balloon has a low profile and folds tightly over a catheter shaft with short shoulders.3 It should dilate to a predetermined diameter at maximal pressure without overexpanding and then deflate rapidly so as to assume its original native configuration for retrieval. The effectiveness of angioplasty is caused by appropriately tearing tunica intima and distention of the medial vascular layer,4 which at times requires high-pressure balloons usually made of thicker material and having larger profiles. The suspect vessel is visualized on angiogram using contrast injection with biplane imaging profiling the lesion to allow for accurate calibrated measurements of length and diameter, as well as any important side branches. Occasionally, multiple angles are needed to best profile a vascular stenosis, especially if it is located adjacent to a superimposed larger structure (Figure 23–1). The standard practice of using the French size of a catheter as a measurement reference should be avoided. Whereas this technique is acceptable for comparable coronary diameters, the larger sizes of major vessels involved in CHD render this technique too inaccurate. A 10% error in the reference measure could result in a miscalculation of several millimeters. Choosing the proper frame for measurement is also important. Although it is natural to select the frame with the best contrast outlining the target vessel, it is actually more important to select the frame with the largest diameter. Because of the pulsatility of some vessels, there may be a significant difference between the largest and smallest diameters during systole and diastole of a cardiac cycle (Figure 23–2). This is especially true for systemic arteries such as the aorta. Introduction of dynamic angiography and three-dimensional (3D) rendering adds another important modality for better understanding the anatomy before an intervention. Figure 23–1 A, Bilateral pulmonary artery stenosis in anteroposterior projection; left pulmonary artery (LPA) stenosis (arrows) is not well seen because of superimposition of the dilated main pulmonary artery. B, At 30 degrees left anterior oblique/30 degrees cranial angulation, the LPA orifice stenosis is profiled. Figure 23–2 Proper frame selection is needed to accurately measure the diameter of a pulsatile vessel. Once measurements are made, the lesion is crossed with an end-hole catheter and placed as far distal as possible. In adults with branch pulmonary artery stenosis and a dilated right atrium and ventricle, passage of any catheter across the right heart may be challenging and require all kinds of techniques to avoid catheter and wire buckling within the atrium or ventricle. A very useful technique is to shape a “left pulmonary artery (LPA)” or “right pulmonary artery (RPA)” curve with the stiff end of a guidewire to help guide the catheter into position. Once this is achieved, a stiff wire is introduced in a manner maximally accommodated by the lumen of the balloon catheter so as to allow for appropriate support and maintain balloon position for dilation. A commonly used wire is an Amplatz Super Stiff “short tip” (Boston Scientific, Natick, Mass.). These have only a 2-cm floppy tip at the end to maximize the length of stiff wire crossing the right ventricular outflow tract. The chosen length and diameter of a balloon that will not impinge on smaller caliber vessels undergoes a “negative prep” to remove all air while not altering the tight factory folding with a large syringe of 1:3 to 1:5 dilute contrast/saline, a three way stopcock, and a pressure monitor inflation device. The larger the balloon, the less concentration of contrast to saline is needed to visualize the inflation, and the quicker the inflation and deflation. Clinical use of intravascular stents has grown since its early use in peripheral and branch pulmonary arteries in humans.5,6 Stents are indicated when vascular elastic recoil or external compression results in restenosis in spite of adequate angioplasty. Since the early days of stenting using the Palmaz stents (Johnson and Johnson, Bridgewater, N.J.), stents are now available in a wide variety of materials, tensile strength, or cell design; self-expanding or balloon-expandable; and in various diameters and lengths. Unlike coronary artery disease, in which most vessels are similar in size, vascular obstructions in CHD can vary widely in location and size and present in all ages from infancy to adulthood. Hence it is prudent to use a stent that can be serially dilated to the normal adult size of the vessel in which it is implanted. After accurate measurements are made of the lesion to be stented, an end hole is introduced past the area of narrowing and placed far distally. The 0.035-inch Amplatz Super Stiff “short-tip” exchange guidewire is most commonly used because it has only a 2-cm floppy tip that allows for more stiff wire to traverse the stenotic area, which improves the trackability for the stiff long sheath and subsequent stent/balloon catheter combination, especially across dilated chambers in pulmonary artery stenting. Careful attention is paid to the wire tip in the distal small branches to avoid perforation. A balloon with short shoulders is preferred to avoid pinhole perforation by the stent edge, but newer stents with rounded edges have reduced this complication. Predilation before stenting has advantages of allowing the vessel compliance to be tested and making sure the balloon does not slip during expansion, but testing may also reduce the radial force to hold a stent and is an added step that is not necessary for all cases. The chosen sheath should have a French size large enough to accommodate delivery of the balloon and stent (usually 1F to 2F larger than needed for the balloon alone) and long enough to traverse the lesion. Although some have advocated delivery of a premounted stent without long sheaths, this technique carries risks, because there is no sheath to prevent the stent from slipping backwards when the balloon is withdrawn from the stent. The exact stent and balloon size are chosen to allow for relief of the present obstruction but so as to not limit future expansion to appropriate adult vessel caliber. The stent is hand crimped on the balloon using umbilical tape to facilitate even circumferential compression (Figure 23–3). Some interventionists add a small amount of contrast on the stent/balloon unit to enhance “stickiness” and try to minimize any stent shifts while it is advanced through the long sheath. The balloon and stent system are then advanced through the long sheath and positioned over the stiff wire using a roadmap from prior angiograms. Figure 23–3 A stent has been mounted onto a balloon, and umbilical tape is wrapped around the stent once and pulled in opposite directions (arrows). This technique provides even circumferential pressure to crimp the stent and minimize the profile of the stent/balloon unit for delivery. Once the balloon and stent are centered on the lesion with the sheath tip just distal to the lesion, the sheath is withdrawn slowly over the balloon/stent. Exact positioning is ensured by an angiogram from a second catheter, and adjustments are made for appropriate centering of the stent. For initial deployment greater than 15 mm in diameter, the BIB balloon (NuMED, Hopkinton, N.Y.) is ideal. This balloon has an inner and outer balloon for expansion. The inner balloon has a diameter one half the size of the outer balloon and is several millimeters shorter in length. The inner balloon is inflated first, and then the outer balloon is inflated. The design of this balloon allows for a more even stent expansion and minimizes foreshortening in larger diameters. Another advantage of this balloon is that small stent adjustments can be made for optimal positioning even after inner balloon expansion but before outer balloon expansion. Branch pulmonary artery stenoses are now routinely addressed in the catheterization lab. They occur in multiple settings, including discreet or long-segment stenosis in sites of old shunt insertions (Blalock-Taussig, Waterston and Potts), pulmonary artery bands, conduits, or postsurgical repairs. They can occur with geometric aberrations such as folds, kinks, or twists or as a result of external compression by adjacent structures. They can be congenital with multiple stenoses and diffuse hypoplasia (i.e., Williams or Alagille syndrome). In general, indications for relief of obstruction include greater than one half to two thirds systemic right ventricular pressure, hypertension in unaffected portions of the vascular bed, or marked decrease in flow to an affected portion or clinical symptoms.7 More recent American Heart Association (AHA) guidelines8 for angioplasty include: Class I: 1. Pulmonary angioplasty is indicated for the treatment of significant peripheral branch pulmonary artery stenosis (see text for definition of “significant” stenosis) or for pulmonary artery stenosis in very small patients in whom primary stent implantation is not an option (Level of Evidence: B). Class IIa: 1. Pulmonary angioplasty is reasonable to consider for treatment of significant distal arterial stenosis (as defined in the introduction to Pulmonary Artery Angioplasty and Stent Placement) or for stenosis in larger, more proximal branch pulmonary arteries that do not appear to be amenable to primary stent implantation (Level of Evidence: B). Class IIb: 1. Pulmonary angioplasty may be considered for treatment of significant main pulmonary artery stenosis that results in an elevation of pressure to more than two thirds of systemic pressure in the proximal pulmonary artery segment or in the right ventricle (in the absence of pulmonary valve stenosis). This stenosis is usually a form of supravalvar pulmonic stenosis, which is not particularly responsive to balloon dilation alone (Level of Evidence: C). For stent implantation, the AHA guidelines are: Class I: 1. Primary intravascular stent implantation is indicated for the treatment of significant proximal or distal branch pulmonary artery stenosis when the vessel/patient is large enough to accommodate a stent that is capable of being dilated to the adult diameter of that vessel (Level of Evidence: B). Class IIa: 1. It is reasonable to consider pulmonary artery stent implantation in critically ill postoperative cardiac patients when it has been determined that significant branch pulmonary artery stenosis is resulting in a definite hemodynamic compromise in a patient/vessel of any size, particularly if balloon dilation is unsuccessful (Level of Evidence: B). 2. Primary intravascular stent implantation is reasonable in the treatment of significant stenosis of the main pulmonary artery segment that results in elevation of the right ventricular pressure, provided that the stent definitely will not compromise a functioning pulmonary valve and will not impinge on the pulmonary artery bifurcation (Level of Evidence: B). Class IIb: 1. It may be reasonable to implant small pulmonary artery stents that lack the potential to achieve adult size in small children as part of a cooperative surgical strategy to palliate severe branch pulmonary artery stenosis. These stents may need to be enlarged surgically or removed during a future planned operation (e.g., conduit replacement, Fontan completion) (Level of Evidence: C). Balloon dilation remains the preferred treatment of choice in very small infants where a large enough stent to dilate to adult size may be technically difficult if not impossible, in patients with anticipated surgery, and in patients with highly resistant lesions that do not respond to even high-pressure balloons and require specialized cutting balloons. Most of the other lesions are amenable to stenting of the branch pulmonary arteries to allow complete relief of obstruction without eventual recoil (Figure 23–4). The technique for single-vessel stenting is described earlier under the section entitled “General Techniques for Stent Implantation.” Figure 23–4 A, Severe proximal right pulmonary artery (RPA) stenosis. B, Angioplasty of RPA stenosis. C, Postangioplasty angiogram show improvement in stenosis. D, At 4-month follow-up catheterization, angiogram showing restenosis of RPA orifice. E, Angiogram after stenting of RPA. F, 20-month follow-up angiogram showing persistent in-stent patency. Pulmonary artery dilation and stenting, given its circuitous route through the right ventricle, may be one of the most challenging interventional procedures. Because of its complexity and high-risk nature, it is preferred to do these procedures with patients under general anesthesia. Usually two venous sheaths are placed: one for balloon dilation or stent deployment, and another for angiography to assure positioning and as a central line for medications as needed. Once the lesion of interest is engaged by the angiographic catheter, selective biplane imaging is obtained in angulations that permit optimal profiling of the stenosis (i.e., right anterior oblique [RAO]/cranial [CR] for the RPA, left anterior oblique [LAO]/CR or caudal [CAU] for the LPA). For dilation only, the balloon size chosen is 3 to 4 times the narrowing or no more than 120% of the adjacent normal vessel. The dilation balloon is advanced and positioned across the stenosis using an angiographic roadmap. Precise manipulations can be accomplished by advancing the balloon over a fixed wire; advancing the wire slightly allows for the balloon to move back. The balloon is typically inflated to burst pressure or until the waist disappears. If hemodynamics are stable, the balloon is held up for 15 to 30 seconds. It is important to observe the deflation as well to evaluate recoil. The authors routinely save the fluoroscopy of the inflations for future review. A careful review of follow-up angiography is needed postdilation to look for signs of residual stenosis and more importantly, any aneurysms or tears. Some very tight lesions may require special cutting balloons to adequately relieve the waist. Unfortunately, the maximal size of cutting balloons is limited to 8 mm in diameter and for balloon withdrawal to avoid separation of the blades from the balloon. Careful review of the angioplasty is required to measure the diameter of the residual waist. The cutting balloon selected should be no more than 1 to 2 mm larger than the residual waist. The purpose of the cutting balloon is to “score” the intima and media and allow for more effective dilation with larger regular balloons and/or additional stenting (Figures 23–5 and 23–6). Repeated angiography is crucial to evaluate for aneurysms and dissections.
Percutaneous Relief of Vascular Obstruction
Potpourri
23.1 Key Points
23.2 Basic Concepts
Balloon Angioplasty
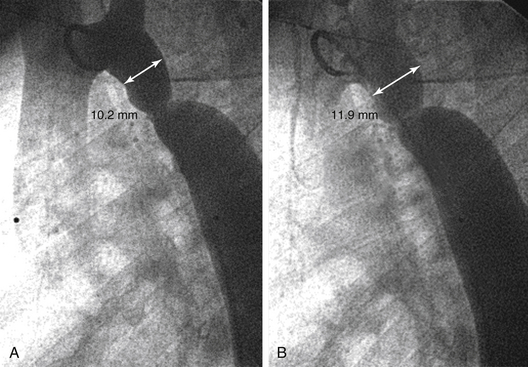
A, Coarctation of the aorta: it is tempting to select the frame with the most contrast. The diameter of this frame measures 10.2 mm (double-headed arrow). B, In the same angiogram, this frame shows less contrast, but the diameter measures 11.9 mm, a 20% increase.
Stenting
General Techniques for Stent Implantation

23.3 Angioplasty and Stenting of Pulmonary Artery Branch Stenosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine