16 Percutaneous Mitral Commissurotomy and Balloon Aortic Valvuloplasty
Percutaneous Mitral Commissurotomy
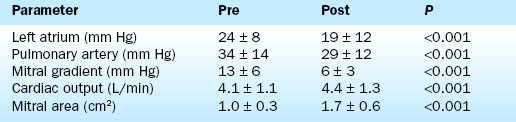
Hemodynamic results have been well characterized by the Inoue Multi-Center Registry. On average there is more than 80% increase in mitral valve area. Balloon inflation results in splitting of the fused commissures with reductions in the transmitral pressure gradient, the mean left atrial pressure, and the pulmonary artery pressure. The cardiac output and mitral valve area increase (Table 16-1).
The most important complication of the procedure is mitral regurgitation. Mitral valve replacement is needed during the initial hospitalization in about 2% of patients. An additional 3% to 4% have resultant 3+ or greater mitral regurgitation without the need for immediate valve replacement. Other complications are shown in Table 16-2.
Table 16-2 Complications of Percutaneous Transvenous Mitral Commissurotomy
| Complication | % |
|---|---|
| Hospital mitral valve replacement (MVR) | 1.0 |
| Hospital death | 1.4 |
| Transient ischemic attack | 0.6 |
| Stroke | 0 |
| Cardiac perforation | 1.4 |
| Pericardiocentesis | 1.0 |
| Myocardial infarction | 0.3 |
| Cardioversion shock for atrial or ventricular fibrillation | <1 |
| Vascular repair | 0.6 |
| Transfusion | 0.3 |
| Temporary pacer | 0 |
| Mitral regurgitation 3+ or more (no MVR) | 3.8 |
| Atrial septal defect >1.5 | 3.1 |
| Failure to cross mitral valve | 1.7 |
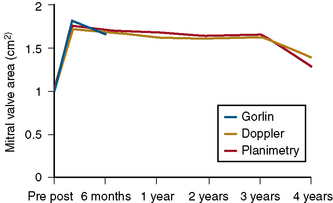
The durability of the results is excellent. Figure 16-1 shows the stability of the achieved valve area over a period of years. The 5-year actuarial freedom from death with mitral valve replacement or repeat balloon commissurotomy for the Inoue Registry population was 71%. More than 80% of the patients remained symptomatically improved at 5 years.
Technique
The Inoue Balloon
The Inoue device differs substantially from conventional balloons. It is constructed of two layers of latex with a nylon mesh sandwiched in between them. The latex is compliant, whereas the nylon mesh limits the maximum inflated diameter of the balloon and gives it a unique shape and three-stage inflation characteristics (Fig. 16-2).
Patient Evaluation
Evaluation by two-dimensional transthoracic and transesophageal echocardiography is essential before mitral valvotomy. Patients with thin, pliable mitral leaflets and minimally diseased subvalvular apparatus have the best long-term outcome from surgical commissurotomy (Fig. 16-3). This is no less true when using percutaneous methods to achieve commissurotomy. Although the immediate results of percutaneous transvenous mitral commissurotomy (PTMC) are acceptable in patients with significant valve deformity, the restenosis rate and the need for rate mitral valve replacement remains higher in these patients. The goal of therapy and the long-term prospects for event-free survival must be appropriate for patients with significant valve deformity and echocardiographic scores greater than 10 to 12.
Cardiac Catheterization Technique
1. The left femoral arterial and venous sheaths are placed. Because a pigtail catheter will be left in place in the left ventricle for a relatively long period of time, we prefer to use 5F or 6F arterial catheters.
2. A multilumen pulmonary artery balloon catheter with thermodilution cardiac output capability is used for right heart catheterization. Left femoral access is preferred for these catheters, leaving the right side for insertion of the dilatation balloon catheter. Pulmonary artery catheters with oximetric monitoring simplify the evaluation of venous saturations for the detection of atrial shunting following the procedure, although these catheters are more difficult to place than are conventional pulmonary artery catheters. Passage of the pulmonary artery catheter is facilitated by the use of an extra-stiff 0.025-inch guidewire.
3. Left ventriculography and coronary arteriography are performed when indicated. The AHA/ACC guidelines for valvular heart disease recommend arteriography for men over age 35 years, or women over age 35 years who also have risk factors.
4. Right heart pressures and cardiac output are measured.
5. Right femoral venous puncture is performed for placement of an 8F Mullins sheath. Placement of a 14F sheath at this stage makes passage and removal of the balloon much easier. An extra-stiff 0.035-inch wire should be used for insertion of the large venous sheath. If a 14F sheath is not used, free movement of the balloon can be impaired by binding in the subcutaneous tissues at the groin puncture site. In very heavy patients the catheter may make a severe angle between the skin and the femoral vein. An ipsilateral pulmonary artery catheter does not interfere with the performance of the transseptal catheterization.
6. Following transseptal puncture, heparin is administered. The transmitral pressure gradient is measured using the Mullins sheath for the left atrial and the pigtail for left ventricular pressures. If the Mullins sheath can be passed into the left ventricle with a gentle counterclockwise rotation, a transaortic gradient is measured with the Mullins and pigtail to exclude aortic valve disease.
Selection of Balloon Size
The maximum expected inflated balloon diameter may be selected based on the patient’s height (Table 16-3). This value provides a guideline for balloon selection with a stepwise technique. A first inflation is always performed at a diameter smaller than the maximum possible for the selected balloon. An initial inflation of 2 to 4 mm less than the maximum is usually chosen. An alternative method for selecting balloon size is to calculate the ratio of inflated dilating balloon area to the body surface area, called the effective balloon dilating area (EBDA).
Table 16-3 Selection of Balloon Size for Percutaneous Transvenous Mitral Commissurotomy
| Balloon diameter (range, mm) | Balloon dilating area (cm2) | Patient height, cm (inches) |
|---|---|---|
| 26 to 30 | 7.07 | >180 (70.9) |
| 24 to 28 | 6.16 | >160 (62.9) |
| 22 to 26 | 5.13 | <160 |
Special Considerations
Balloon Preparation
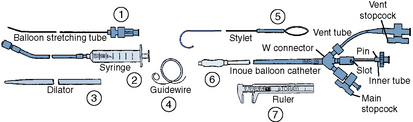
Once the diagnosis of mitral stenosis is confirmed after successful transseptal puncture, the balloon catheter can be prepared. The balloon catheter comes packaged with all the components necessary for the dilatation procedure (Fig. 16-4). These include:
• A balloon-stretching metal tube
• A calibrated inflation syringe specifically matched to each balloon
• A rigid 12F to 14F plastic dilator
• A 0.025-inch spring-tipped exchange guidewire
• A stylet for manipulating the balloon across the mitral valve after it has been placed in the left atrium
• Calipers for measuring the balloon diameter and confirming its inflated size
Balloon Valvotomy
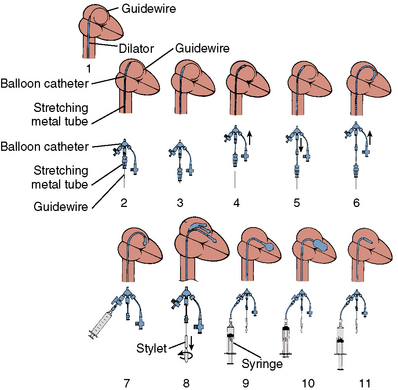
The major steps in valvotomy are illustrated diagrammatically in Figure 16-5. The 0.025-inch spring guidewire is advanced through the Mullins sheath into the left atrium with the fully coiled distal portion out of the sheath and positioned in the roof of the atrium. The Mullins sheath is withdrawn over the guidewire with the guidewire remaining in the left atrium. The dilator is advanced through the skin and then into the atrial septum, where it may be passed through the septal puncture as shown in Figure 16-5. The dilator is left sitting in the septal puncture for several seconds to stretch the septal tissue. The dilator is removed and the balloon catheter is passed over the guidewire via the 14F sheath and then across the atrial septum.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree