(1)
Department of Interventional Cardiology, Otamendi Hospital, Buenos Aires, Argentina
(2)
Cardiac Unit, Department of Interventional Cardiology, Revista Argentina de Cardioagiologia Intervencionista (RACI), Otamendi Hospital, Buenos Aires, Argentina
(3)
Interventional Cardiology Unit, Department of Interventional Cardiology, Otamendi Hospital, Buenos Aires, Argentina
(4)
Otamendi Hospital, Post Graduate Buenos Aires School of Medicine, Buenos Aires, Argentina
(5)
Cardiovascular Research Center (CECI), Buenos Aires, Argentina
Abstract
Left main disease (LMD) is a stenosis of ≥50 %, and occurs in 3–5 % of patients, associated with multi-vessel coronary artery disease (CAD) in more than 75 %. With bare metal stents (BMS), left main stenting became more popular and the technique was used mainly in high risk patients such as acute myocardial infarction (AMI) or with contraindications for coronary artery bypass grafts (CABG). There was an initial low incidence of adverse events with this approach, but with concerns regarding restenosis-related events, drug eluting stents (DES) became the preferred option. Comparative studies with CABG and DES showed that both had similar mortality and incidence of MI but with higher recurrence of repeat revascularization procedures with DES. CABG had a higher stroke risk. Percutaneous Coronary intervention (PCI) should be a good option in most patients with LMD without bifurcation disease, in those with a small diameter circumflex artery and /or low or intermediate anatomic risk score. The latest European guidelines recommended PCI with DES implantation as a class I indication in certain subgroups of patients with LMD. On the other hand, CABG is still the “gold standard” and has remained a better option when the SYNTAX score is ≥33, with severe multi-vessel coronary disease, total occlusions of ≥2 major coronary epicardial vessels, severe calcifications or tortuosity, and in those with a contraindication to antiplatelet therapy.
Keywords
Coronary diseaseLeft mainBare metal stentsDrug eluting stentsBifurcation lesionsCoronary by pass graftRegistryRandomized trialMeta analysisIntroduction
Significant left main disease (LMD) is defined as a stenosis of ≥50 %, and is associated with multi-vessel coronary artery disease (CAD) in more than 75 % of patients. LMD occurs in 3–5 % of patients undergoing coronary angioplasty [1]. Coronary artery bypass graft surgery (CABG) has remained as the standard of care therapy for patients with LMD mostly because the long-term outcomes, including mortality, were superior to those of medical treatment [2, 3].
Percutaneous coronary intervention (PCI) in LMD was first performed during the balloon angioplasty era although because of the risk of acute closure, this became a contra indication to PCI. With the introduction of bare metal stents (BMS) during PCI, left main stenting became more popular and the technique was reported in observational studies and registries mainly in high risk patients such as those with acute myocardial infarction (AMI) or with contraindications for myocardial revascularization with CABG [4, 5]. Observational studies reported an initial low incidence of adverse events with this approach [4] since BMS lowered the incidence of abrupt vessel closure, but concerns arose regarding high rates of restenosis-related events particularly for those lesions located distally and involving the bifurcation. Drug eluting stents (DES) have significantly decreased the risk of restenosis and target lesion revascularization (TLR) compared to BMS. Therefore, their introduction routinely during PCI has enhanced the mid and long -term outcomes of PCI for LMD [5, 6]. In this chapter, we will discuss the most relevant aspects about PCI in LMD analyzing mid and long term results from observational studies as well as randomized trials and meta-analyses, focusing studies mainly on DES designs.
PCI with Stenting
DES Versus BMS in Observational Studies and Registries
There are several observational studies comparing BMS and DES in LMD. In most, event free survival was improved with DES. In the LE MANS [7] (Left MaiN coronary artery Stenting) registry, performed in Poland, 252 patients were enrolled during 11 years between 1997 and 2008 with 58 % having a non ST segment elevation acute coronary syndrome, diabetics in 26.4 % and a distal location of the stenosis in 56 % [8]. Between 1997 and 2001 only BMS were used. After that, DES was recommended for the LM with a reference diameter ≤3.8 mm. First generation DES [paclitaxel (PES) and sirolimus (SES)] were implanted in 36.2 % of patients. The Euro score surgery risk was 6 ± 2.
Major adverse cardiovascular and cerebral events (MACCE) defined as death, myocardial infarction (MI), TLR, stent thrombosis (ST) or cerebrovascular accident (CVA) stroke occurred in 12 (4.8 %) patients during the 30-day follow-up period. During long-term follow-up (mean 3.8 years, range 1–11 years) MACCE rates were 25.4 % and 13.9 % of patients died. The 5- and 10-year survival rates were 78.1 % and 68.9 % respectively. Despite more favorable baseline characteristics in patients treated with BMS, unmatched analysis showed a significantly lower MACCE rate in DES patients (25.9 % vs. 14.9 %, respectively p = 0.039). This difference was strengthened even further after propensity score matching. DES lowered both mortality and MACCE for distal LMD lesions when compared to BMS. Ejection fraction <50 % was the only independent risk factor influencing long-term survival.
The Italian Society of Invasive Cardiology ran a multicenter retrospective registry [9] in 19 high volumes centers enrolling 1453 consecutive patients who underwent PCI on LMD between January 2002 and December 2006. Four hundred and seventy-nine received either first generation DES (334 patients) or BMS (145 patients) but only for lesions located at the ostium or shaft, without any distal involvement. After propensity score matching was performed, baseline covariates between the two yielded 119 well-matched pairs. At 3-year follow-up, risk-adjusted survival rates were higher in patients treated with DES than in those treated with BMS, although the adjusted 3-year rates of TLR were not significantly lower with DES compared to BMS (P = 0.60).
Our group began to perform left main stenting in the early 1990s soon after PCI with BMS became a default strategy [4]. In 1996, the ERACI II study [10, 11] became the first randomized revascularization trial that included unprotected LMD patients. This included about 5 % of the overall population, 4 % in CABG and 5.8 % in the PCI arms. Moreover, in Argentina in 2013, a multicenter registry was published in patients with unprotected LMD [12]. Two hundred and eighty-one consecutive patients treated between 2002 and 2012 were included. Baseline characteristics included mean age of 67.1 years, diabetes in 18.5 %, a Euro SCORE of 5.5, acute coronary syndromes in 72.9, 25.4 % of them with ST elevation MI (STEMI) and distal LMD stenosis in 49.8 %. 391 stents were implanted, BMS and DES in 49 % and 51 % respectively. During a mean follow-up 3.2 ± 2.7 years, death occurred in 9.6 and 6.0 % had an MI; 14.5 % had death/MI/CVA and 30 % MACCE (Fig. 16.1). Compared to proximal lesions, distal lesions had a significantly worse freedom from death/MI/CVA (81.9 % vs. 89 %, respectively = 0.012) (Fig. 16.2) and MACCE (66.7 % vs. 77.7 %, respectively p = 0.02). Patients receiving a DES had less MACCE than with BMS (p = 0.025). Those treated with BMS also had poorer outcomes with distal lesions (Fig. 16.3). Better event free survival with DES was also observed in patients treated for an acute MI with ostial LAD or LCX involvement. Thus, DES implantation had become the default strategy in left main PCI [13]. Further pooled data from several trials suggested better outcomes with DES compared to BMS. A meta-analysis [14] with 10,342 patients from 44 studies assessed the incidence of death/MI, TVR/TLR and MACCE at 3 years. Mortality (8.8 and 12.7 %, p = 0.01), TVR/TLR (8.0 and 16.4 % with DES and BMS: p = 0.01), and MACCE (21.4 and 31.6 %, p = 0.12) were all lower with DES.
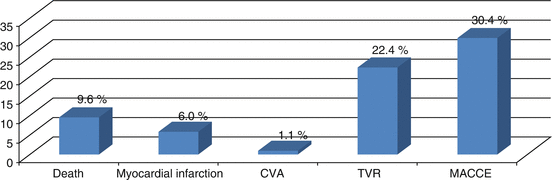
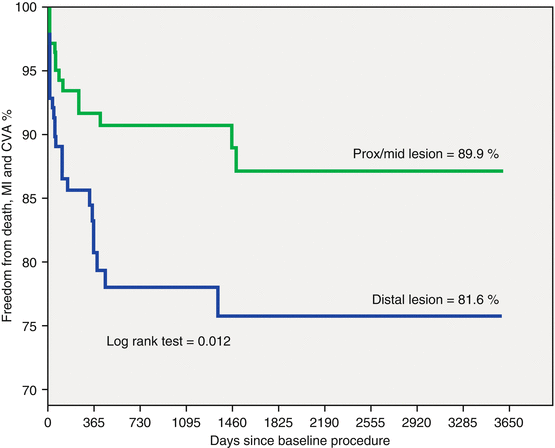
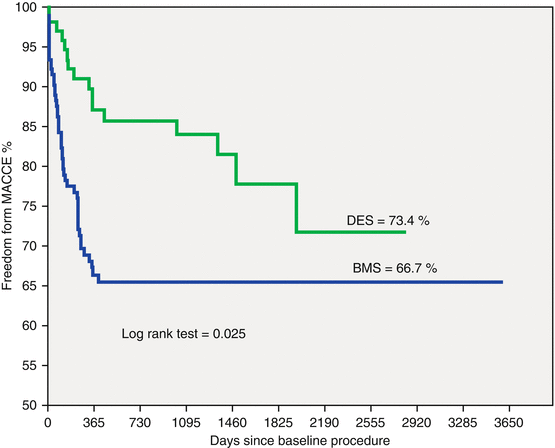

Fig. 16.1
Left main Argentine CECI registry, overall results at 3.2 years of follow-up (mean). CECI Cardiovascular Research Center, CVA cerebrovascular accident, TVR target vessel revascularization, MACCE major adverse cardiovascular events: death, MI, CVA and TVR

Fig. 16.2
Kaplan-Meier Death, myocardial infarction and cerebrovascular accident curve at 3.2 ± 2.7 years follow- up comparing distal vs. proximal lesions (n = 281). Prox/mid proximal and/or mid left main coronary lesions. MI myocardial infarction

Fig. 16.3
Kaplan-Meier MACCE survival curve at 3.2 ± 2.7 follow-up comparing DES vs. BMS. MACCE major adverse cardiovascular events (death, myocardial infarction, cerebrovascular accident and target vessel revascularization); DES drug eluting stent, BMS bare metal stent
DES Stent Selection
There are few studies that compared different DES designs in LMD. A randomized trial [14] and two observational studies [15, 16] suggested that outcomes were comparable between both first generations DES. However, now we are using second and third generation drug- (limus family mostly, sirolimus, everolimus, zotarolimus and biolimus) eluting stents with new polymers, including biocompatible or biodegradable polymers. In the Florence registry, 390 patients underwent PCI with DES implantation, 224 received a paclitaxel eluting stent (PES) and 166 an everolimus-eluting stent (EES) [17]. At 9 months, coronary restenosis by angiography was 5.2 % in EES and 15.6 % in PES (p = 0.002). Among 166 propensity matched pairs, the rate of MACCE at 2 years was significantly higher with PES (20.4 versus 10.2 %, respectively, p = 0.010).
In the Left main Taxus and left main Xience (LEMAX) non-randomized registry, 173 patients treated with an EES were compared with a historical cohort of 291 patients treated with PES. At 12-month clinical follow-up, the EES was associated with a lower rate of target lesion failure, MACCE and ST compared with PES [18]. The ISAR-LEFT-MAIN 2 study compared the safety and efficacy of the zotarolimus-eluting stent (ZES) versus EES [19]. Patients were randomly assigned to receive either a ZES (n = 324) or an EES (n = 326). The primary endpoint was the combined incidence of death, MI and TLR at 1 year. Secondary endpoints were definite or probable ST at 1 year and angiographic restenosis based on analysis of the left main coronary artery area at follow-up angiography. At 1 year, the cumulative incidence of the primary endpoint was 17.5 % in the ZES group and 14.3 % in the EES group (p = 0.25). Three patients in the ZES group (0.9 %) and 2 patients in the EES group (0.6 %) experienced definite or probable ST (p < 0.99). All-cause mortality at 1 year was equal in the 2 groups (5.6 %). Angiographic restenosis occurred in 21.5 % in the ZES group and 16.8 % in the EES group (p = 0.24). The results suggested that the use of second-generation EES and ZES had comparable outcomes to those noted with the use of first-generation DES, and both stent types appeared to afford similar results at 1-year follow-up.
PCI Versus CABG
Observational Studies
There are several non-randomized studies and registries (Table 16.1) comparing DES to CABG for patients with LMD [21–23]. The DELTA multicenter registry [20] included consecutive “all comers” with LMD treated either with PCI and a 1st generation DES or CABG between April 2002 and April 2006. Patients treated in 14 centers were retrospectively analyzed in this worldwide registry. A propensity score analysis was performed to adjust for baseline differences in the overall cohort. In total 2775 patients were included: 1874 were treated with PCI and 901 with CABG. At 1295 days (range: 928–1713), there were no differences, in the adjusted analysis, in the primary composite endpoint of death, cerebrovascular accidents, AMI, or the composite endpoint of death and AMI . An advantage of CABG over PCI was observed in the composite secondary endpoint of MACCE (p = 0.0001), driven exclusively by the higher incidence of TVR with PCI.
Table 16.1
Randomized clinical trials and registries comparing CABG vs. PCI for the treatment of unprotected left main disease
Trial | Design | CABG | PCI (DES%) | Primary endpoint | Follow-up (primary endpoint) | Remarks | Authors main conclusion |
|---|---|---|---|---|---|---|---|
LEMANS Registry [7] | Observational | 1849 | 252 (37 %) | All-cause of death | 3.8 years (range 1–11 years) | DES decreased the risk of long-term MACCE, particularly in distal lesions. | PCI is feasible and offers good long-term outcome. |
DELTA [20] | Observational | 901 | 1874 (100 %) | All-cause death, CVA, MI and repeat revascularization. | 1295 days (range 928–1713) | It was an all-comers registry | No differences were observed in death, CVA and MI between groups. |
Lee et al. [21] | Observational | 123 | 50 (100 %) | MACCE | 6 months | More than 50 % of high risk patients (Parsonnet >15) | PCI was not associated with an increase in immediate or medium-term complications. |
Chieffo et al. [22] | Observational | 142 | 107 (100 %) | Death; death/MI; death/MI/CVA; repeat revascularization; MACCE | 12 months | In-hospital results were significantly in favor of PCI | There were no differences between groups. |
Seung et al. [23] | Observational | 1138 | 1102 (71 %) | Death; death/QMI or CVA; repeat revascularization. | 1017 (688–1451) days | Even with DES, PCI was associated with higher TVR | No differences in death, MI or CVA. |
MAIN-COMPARE [24] | Observational | 1138 | 1102 (71 %) | Death; death, MI and CVA; and repeat revascularization | 5.2 years (range 3–9 years) | Similar results comparing DES vs CABG and BMS vs CABG | PCI had similar death, MI and CVA with greater rates of TVR |
LEMANS Randomized [25] | RCT | 53 | 52 (67 %) | LVEF | 12 months | Late FU (>2 years) MACCE-free survival was similar in both groups. | LVEF had improved significantly only in PCI arm. |
Precombat [26] | RCT | 300 | 300 (100 %) | All-cause death, CVA, MI, and | Precombat [26] | RCT | 300 < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|