18 Percutaneous Coronary Intervention in Acute ST Segment Elevation Myocardial Infarction
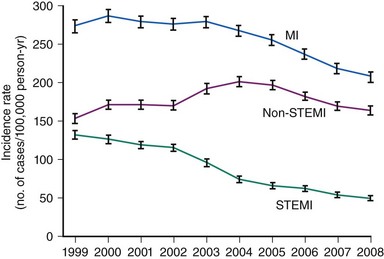
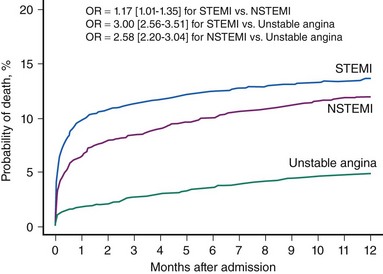
Acute myocardial infarction (MI) can be defined on the basis of a constellation of indices related to clinical, electrocardiographic, biochemical, and pathologic characteristics.1 According to the most recent American Heart Association (AHA) Heart Disease and Stroke Statistics, it is estimated that annually 1.25 million Americans have an acute MI.2 Acute MI results mostly from the acute thrombotic occlusion of an epicardial coronary artery, typically occurring as a consequence of atherosclerotic plaque disruption, fissuring, or erosion that leads to exposure of thrombogenic material (plaque lipid content, collagen, and subendothelial extracellular matrix) to circulating blood. Other causative factors of lesser importance include acute plaque expansion (such as that caused by intra-plaque hemorrhage leading to acute closure without or with minimal thrombus formation), embolism, spontaneous dissection, coronary inflammation, and extracoronary factors. In nearly three quarters of all infarct-related arteries, acute thrombosis appears to occur over atherosclerotic plaques causing mild to moderate coronary obstruction; that is, acute thrombotic closure compromises blood supply to an extensive myocardial area.1 Interruption of coronary blood flow results in myocardial ischemia in the blood-deprived myocardial area, which, if severe enough and of sufficient duration, results in myocardial necrosis. Acute ST segment elevation myocardial infarction (STEMI) represents the most malignant presentation of acute coronary syndromes resulting from acute thrombus-mediated closure of an infarct-related coronary artery. Although current evidence suggests that the incidence of STEMI is trending downward (Fig. 18-1) and the STEMI-related mortality has decreased, acute STEMI still represents a severe condition associated with worse short-term prognosis, compared with other less severe presentations of acute coronary syndromes (Fig. 18-2).3,4 Apart from serving as the pathophysiologic basis for STEMI, acute coronary thrombosis forms the rationale for reperfusion therapy that aims at the prompt restoration of coronary blood flow, which results in myocardial salvage, increased electrical stability, and reduced incidence of fatal ventricular arrhythmias in the acute phase, preserved left ventricular function, and improved short-term and long-term patient survival. Abundant evidence in the past three decades bears witness to the life-saving effect of reperfusion therapy—primary percutaneous coronary intervention (PCI) or thrombolysis—in patients with STEMI. PCI achieves its most impressive life-saving effect in acute STEMI. The objective of this chapter is to summarize recent developments in the field of catheter-based reperfusion therapy for patients with acute STEMI.
 Primary Percutaneous Coronary Intervention Versus Thrombolysis
Primary Percutaneous Coronary Intervention Versus Thrombolysis
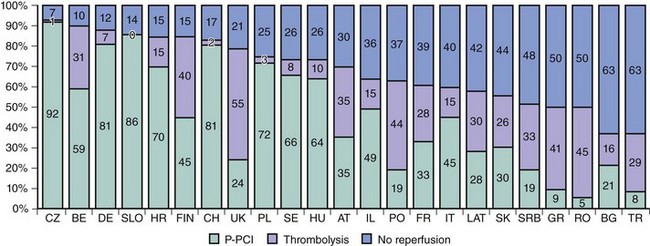
Prompt restoration of coronary blood flow in the occluded coronary artery and the fastest possible myocardial tissue reperfusion form the fundamental principle of early STEMI therapy. Primary PCI refers to a strategy of emergent coronary angiography followed by coronary angioplasty with or without stenting of the infarct-related artery and without prior administration of thrombolytic therapy. In recent years, mechanical reperfusion has become the mainstay reperfusion therapy in patients with STEMI. Current evidence shows that primary PCI has become the dominant reperfusion strategy in patients with STEMI in North America and Europe (Fig. 18-3).5,6 Multiple lines of evidence from randomized studies reported in recent years firmly suggest that catheter-based mechanical reperfusion in patients with STEMI is superior to pharmacologic thrombolytic therapy in terms of improving of early and late survival as well as in reducing the incidence of re-infarction, intracranial bleeding, reocclusion of the infarct-related artery, and recurrent myocardial ischemia.7 Primary PCI is expected to become the “dominant default strategy” for prompt early reperfusion in patients with STEMI if growth and diffusion of resource and logistical factors enable its broader application. Several factors may explain the superiority of primary PCI over thrombolysis. Although thrombolytic therapy has been proven to be life saving, it has serious limitations related to high proportions of patients with relative or absolute contraindications to this therapy; life-threatening bleeding complications disproportionately affecting older patients; a narrow window of therapeutic action after symptom onset because of rapid time-dependent loss of efficacy; limited ability to restore normal blood flow in the infarct-related artery, even if applied in timely fashion; and frequent reocclusions of the infarct-related artery resulting in re-infarction within subsequent months. Apart from being free of these limitations, primary PCI has other advantages over thrombolytic therapy such as restoration of significantly higher rates of thrombolysis in myocardial infarction (TIMI) flow grade 3 in the infarct-related artery (a finding which is both durable because of enhanced stability of the reopened vessel and relatively independent of time from symptom onset) (Fig. 18-4), greater amount of salvaged myocardium, delineation of coronary anatomy and hemodynamic status resulting in risk stratification, and facilitation of patient care and earlier hospital discharge.8 Lack of availability of care centers equipped to offer timely catheter-based invasive treatment to patients with STEMI, rather than issues pertaining to clinical effectiveness, is currently perceived as the most important factor that impedes a broader application of this treatment to patients with STEMI. Recent American College of Cardiology/American Heart Association/European Society of Cardiology guidelines define primary PCI as a class I indication (level of evidence: A) in patients with STEMI (including true posterior MI and MI with new or presumably new left bundle branch block [LBBB]) presenting to a hospital with PCI capability if performed within 90 minutes of the first medical contact.1,9,10 A class I indication (level of evidence: B) is recommended for patients with cardiogenic shock and contraindications to fibrinolytic therapy, irrespective of amount of delay.1 Numerous additional lines of evidence further establish the superiority of primary PCI over thrombolysis. Primary PCI improves survival even in patients with STEMI who have contraindications to thrombolytic therapy or in patients presenting outside the therapeutic window of thrombolysis.11–13 Available evidence suggests that primary PCI is superior to thrombolysis in all studied time intervals. A recent report from the Primary Coronary Angioplasty versus Thrombolysis (PCAT)-2 Trialists’ Collaborative Group demonstrated that primary PCI was associated with a 67% reduction in 30-day mortality compared with thrombolysis, which, in absolute terms, translates into 53 lives saved per 1000 patients treated. For PCI-related delays up to 120 minutes, primary PCI was associated with a 26% reduction in mortality compared with thrombolysis or in 19 lives saved per 1000 patients treated (Fig. 18-5).14 The absolute reduction in mortality with primary PCI widened over time from 1.3% within the first hour to 4.2% after more than 6 hours after symptom onset.14 The superiority of primary PCI over thrombolysis has been observed particularly in high-risk patients and in centers without on-site cardiac surgery.15,16 The DANish trial in Acute Myocardial Infarction-2 (DANAMI-2) demonstrated that the benefit of PCI is largest in high-risk patients: In patients with TIMI risk scores 5 or greater, there was a significant reduction in the mortality with primary PCI (25.3% vs. 36.2% with thrombolysis; P = 0.02), which was not observed in the low-risk group (TIMI risk score 0 to 4).15 The benefit of primary PCI over thrombolysis was maintained at long-term follow-up. A further report from DANAMI-2 showed that the 8-year composite of death or re-infarction was 34.8% in patients treated with primary PCI versus 41.3% in patients treated with thrombolysis (P = 0.003). Of note, primary PCI reduced the risk of re-infarction (13% vs. 18.5%) and mortality (26.7% vs. 33.3%) among patients randomized at referral hospitals.17 The study reinforced the recommendation that primary PCI should be offered to STEMI patients if transport to invasive care centers can be completed within 120 minutes. A recent meta-analysis of 23 randomized and 32 observational studies reported significant improvement in mortality, stroke, and re-infarction with the use of primary PCI compared with thrombolysis.18 Current evidence also points out the cost-effectiveness of primary PCI compared with thrombolysis.19 There is evidence that the use of thrombolytic agents as a reperfusion strategy in patients with STEMI has markedly decreased. Recently, the Global Registry of Acute Coronary Events (GRACE) enrolled, between April 1999 and June 2006,10,954 patients with STEMI or LBBB, who presented within 12 hours of symptom onset; the report from this study documented that the use of primary PCI increased from 15% to 44% and the use of thrombolytic agents decreased from 41% to 16% as reperfusion strategy (Fig. 18-6).20 At present, it seems that the era of comparative studies between mechanical reperfusion (primary angioplasty or stenting) and pharmacologic thrombolytic therapy is over and the superiority of primary PCI over thrombolysis is almost unanimously accepted. In aggregate, these studies demonstrated that primary PCI is superior to thrombolysis and should be the preferred strategy of reperfusion in patients with STEMI in all clinical situations and patient subsets. However, because the majority of hospitals do not have PCI capability, physicians and hospitals are faced with the challenge of providing timely primary PCI to patients with STEMI.21 Recognizing the importance of the problem, several regions in the United States have proposed or established triage and transfer protocols to direct patients with STEMI to hospitals with PCI capability without any delay.22,23 A series of prehospital timing benchmarks have been proposed, the achievement of which is associated with favorable system performance, that is, achieving an interval from first medical contact to PCI of 90 minutes or less.24 Available evidence demonstrates significant improvements in the timely provision of reperfusion therapy to patients with STEMI, the safety and efficacy of primary PCI procedures, and the overall management of patients with acute MI.25
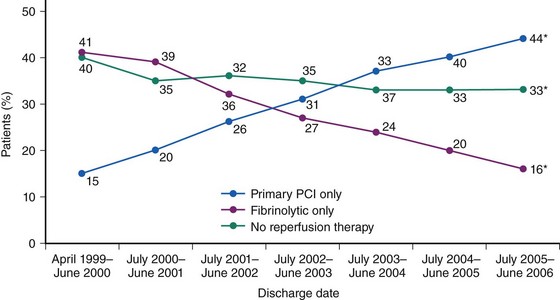
Figure 18-6 Trends in the use of reperfusion therapy in patients with acute myocardial infarction from 1999 to 2006.
(Eagle KA, Nallamothu BK, Mehta RH, et al: Trends in acute reperfusion therapy for ST-segment elevation myocardial infarction from 1999 to 2006: We are getting better but we have got a long way to go, Eur Heart J 29:609–617, 2008.)
 Time-to-Treatment Interval and Outcome
Time-to-Treatment Interval and Outcome
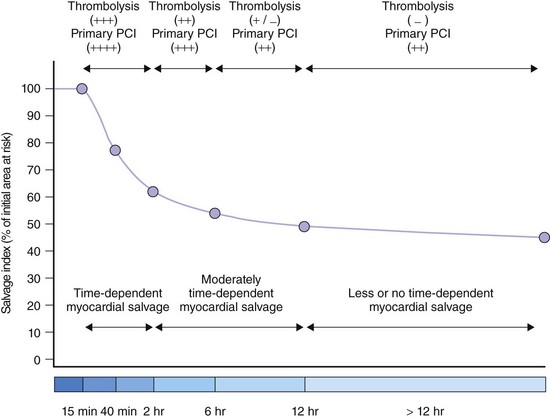
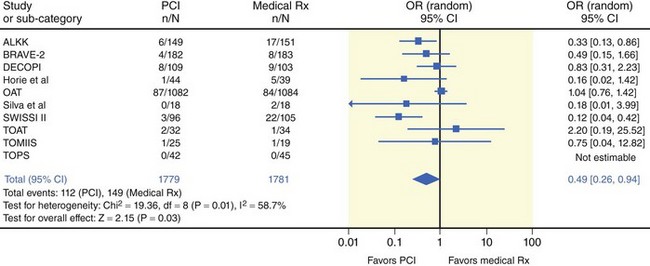
Time-to-treatment interval is an estimate of overall duration of myocardial ischemia that encompasses the period from the onset of symptoms of coronary occlusion to the initiation of reperfusion therapy—thrombolysis or primary PCI. The time-to-treatment interval has two components: (1) the time from symptom onset to patient arrival at the hospital and (2) the time from patient arrival to the initiation of reperfusion therapy. Apart from disclosing the duration of myocardial ischemia, time-to-treatment interval is an index of the quality and readiness of the health care system to provide reperfusion therapy in timely fashion. Knowledge of the biology of myocardial ischemia and the speed with which it leads to necrosis is important to any understanding of the time-dependent efficacy related to reperfusion regimens and the degree of the benefit of reperfusion therapy in general. Abrupt coronary occlusion results in the drastic reduction of coronary blood flow and in myocardial necrosis that progresses gradually and is typically complete in about 6 hours after the onset of occlusion. A rapid phase of cell death, mostly in the subendocardial region, follows the coronary occlusion; about half the ischemic myocardium that is necrotic at 24 hours has already died at 40 minutes after coronary occlusion.26 A second phase of cell death occurs more slowly in the mid-epicardial myocardium and the subepicardial myocardium. This phase of myocardial necrosis is complete within 6 hours of coronary occlusion and about one third of ischemic myocardium is salvageable at 3 hours.26
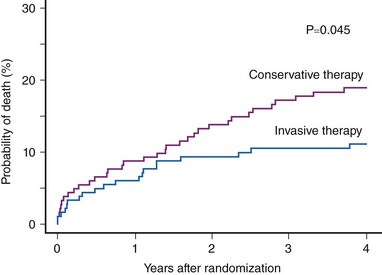
There are, however, considerable differences between coronary occlusion and acute MI in the experimental setting and spontaneously occurring coronary occlusion and STEMI in the clinical setting. Factors such as the often-stuttering course of coronary occlusion, spontaneous recanalization, and persistence of anterograde blood flow in the infarct-related artery, collateral circulation, preconditioning, postconditioning, metabolic need at the time of coronary occlusion, and the effects of initial anti-ischemic (anti-thrombotic) therapy may modify the time-course of the progression of myocardial ischemia to necrosis and may extend the time during which the ischemic myocardium remains viable. Scintigraphic studies have identified viable myocardium in patients with STEMI presenting late (>6 hours) after symptom onset; this viable myocardium appears salvageable if PCI is used as a reperfusion strategy. These studies suggest the existence of two phases of myocardial salvage as well: (1) an early phase of myocardial salvage enabled by early reperfusion (ideally within the first hour) and (2) a late phase of myocardial salvage enabled by later reperfusion. During the early phase, reperfusion is associated with substantial and time-dependent myocardial salvage. However, reperfusion therapy within the first hour following coronary occlusion, which is expected to lead to large amounts of myocardial salvage and the best clinical outcome, is achievable in a very limited number of patients. A prior study has shown that the time-to-treatment interval was less than 2 hours in only 27% of the patients and less than 1 hour in only 3.2%.27 Reperfusion in the second (later) phase results in a lesser degree of and less time-dependent myocardial salvage. Analysis of the time-to-treatment interval in various studies suggests that most patients are reperfused beyond the golden hour of reperfusion, that is, in the late phase of myocardial salvage, in which a slower progression of the ischemic myocardium to necrosis occurs because of the presence of residual blood flow, collaterals, or other factors that widen the window of myocardial salvage. Nevertheless, these data do not mean that myocardial salvage by primary PCI is not time dependent. They merely indicate that the time window for myocardial salvage is wider with primary PCI than with thrombolysis (Fig. 18-7). Time from onset of symptoms to initiation of reperfusion is an important predictor of myocardial salvage and clinical outcome, whether reperfusion is achieved with thrombolysis or primary PCI. However, the dependence of myocardial salvage or clinical outcome on the time-to-treatment interval in patients with STEMI undergoing PCI still remains controversial. Scintigraphic studies provide valuable information on infarct size and allow assessment of the efficacy of reperfusion in terms of myocardial salvage with a high prognostic value (Fig. 18-8). The BRAVE-2 (Beyond 12 hours Reperfusion AlternatiVe Evaluation) trial demonstrated the potential of primary PCI to salvage the myocardium and reduce the infarct size even in patients with STEMI presenting 12 to 48 hours from symptom onset. The median infarct size was 8% in patients with STEMI who were assigned to primary PCI versus 13% in patients assigned to conservative treatment (P < 0.001).13 A recent update from the BRAVE-2 trial demonstrated a mortality benefit for up to 4 years in patients assigned to invasive treatment (11.1% in patients assigned to invasive treatment vs. 18.9% in patients assigned to conservative therapy; P = 0.047; Fig. 18-9).28 Another recent study involving 396 patients with STEMI demonstrated that late comers (presenting >12 hours from symptom onset) had larger infarct size (14% vs. 7%; P = 0.005), lower proportion of initial area at risk salvaged (53% vs. 69%; P = 0.05) and lower left ventricular ejection fraction (LVEF; 44% vs. 57%; P = 0.04) compared with patients presenting within the first 12 hours from symptom onset. Of note, this study showed that substantial myocardial salvage (more than 50% of initial area at risk) occurred in 41% of late presenters.29 Together, these studies demonstrated that in patients with STEMI presenting more than 12 hours from symptom onset, a substantial amount of myocardial salvage occurs when primary PCI is used as the reperfusion strategy. A recent meta-analysis of 10 randomized trials (including the Occluded Artery Trial [OAT]) with 3560 patients with acute MI presenting between 12 hours to 60 days after symptom onset demonstrated significant reduction in long-term mortality (6.3% vs. 8.4%, Fig. 18-10). Eight of these 10 studies showed improvements in long-term survival. There was a greater improvement in LVEF over time in patients who received invasive treatment (+4.4% change in LVEF) compared with patients who received medical therapy.12 With regard to the impact of the time-to-treatment interval on clinical outcome, clinical studies have produced conflicting results. A progressive increase in 1-year mortality with every 15-minute increase in the symptom onset-to-balloon time has been recently reported.30 Conversely, data from the National Registry for Myocardial Infarction (NRMI) showed no association between symptom onset-to-balloon time and survival in a cohort of 27,080 consecutive patients with acute MI treated with primary angioplasty.31 Similarly, an analysis of 2635 patients enrolled in 10 randomized trials of primary angioplasty versus thrombolytic therapy demonstrated that with increasing time-to-presentation interval, major adverse cardiac event rates increased after thrombolysis but remained relatively stable after angioplasty.32 A recent publication of the DANAMI-2 trial, involving only the primary PCI substudy showed that 3-year mortality did not differ among patients with symptom onset-to-balloon times greater than 3 hours and 3 to 5 hours.33 However, the mortality rate was significantly higher in patients presenting 5 hours or later from symptom onset (hazard ratio [HR] 2.36; P < 0.001), and the difference in mortality rates remained significant after adjustment for potential confounders. A shorter symptom onset-to-balloon interval was associated with greater rates of TIMI flow grade of 3 after primary PCI and with lower proportions of patients with a LVEF of 40% or less.
Knowledge of the risk distribution alongside the time-to-treatment interval is of importance in understanding the time-dependent efficacy of primary PCI in patients with STEMI. Prior studies have shown that patients presenting early after symptom onset have the highest risk score, which is consistent with the observation that early presenters have the largest cumulated ST-segment elevation, reflecting the largest initial areas at risk and prompting urgent seeking of medical aid.34,35 This group of patients with STEMI benefit mostly from primary PCI in terms of myocardial salvage, preservation of ventricular function, and survival because of early intervention and the characteristics of the ischemic lesion. Patients who present later after symptom onset may have a smaller initial area at risk producing milder symptoms, and their outcome may be influenced by the survivor–cohort effect; that is, they have already survived the highest risk of death in the early hours after coronary occlusion. Late presenters may have also a more adverse cardiovascular risk profile. Prior reports show that patients who presented later were older, more often women and diabetic, and had a past history of coronary bypass surgery. Adjusting for these factors considerably attenuates the association between the time-to-treatment interval and mortality (from highly significant in univariable analysis to a borderline significance after adjustment in multivariable model).30 Patients with a greater delay in admission are also expected to present more frequently with additional adverse characteristics such as impaired renal function, peripheral arterial disease, and greater inflammatory burden—factors not accounted for in the multivariate model. The lack of adjustment for these characteristics further widens the gap between statistical and biologic adjustments with regard to prognosis. Associated comorbidities and the less favorable cardiovascular risk profile may mask the benefits of mechanical reperfusion from myocardial salvage, and the unfavorable outcome after coronary intervention may erroneously be attributed solely to the longer time-to-reperfusion interval. Therefore, it is highly probable that a more adverse baseline risk profile of patients with longer delay to presentation may explain, at least in part, the apparent association between the time-to-treatment interval and mortality. These considerations are important because the apparent reduction of benefit from PCI with increased time to presentation may be interpreted as a poor incentive for prompt intervention in patients with delayed presentation who are badly in need of this treatment.
Although the superiority of primary PCI over thrombolysis for longer time-to-presentation intervals is undeniable, whether primary PCI is superior to thrombolysis when applied early after symptom onset (within 2 hours) is still debated. Most of this controversy was generated after the publication of a subset analysis from the Comparison of Angioplasty and Prehospital Thrombolysis In acute Myocardial infarction (CAPTIM) trial involving 460 patients; this trial demonstrated that patients with STEMI randomized less than 2 hours after symptom onset had a trend toward lower 30-day mortality with thrombolysis compared with primary PCI (2.2% vs. 5.7%, P = 0.058).36 The reliability of these results is strongly compromised by the fact that they were produced by a subset analysis of a prematurely terminated trial. In fact, a recent meta-analysis by the Primary Coronary Angioplasty versus Thrombolysis (PCAT)-2 Trialists’ Collaborative Group based on individual patient data has demonstrated that primary PCI is superior to thrombolysis in terms of reduction of the 30-day mortality even for a presentation delay of less than 2 hours as was the case for 2747 patients (4.3% with PCI vs. 6.2% with thrombolysis, P = 0.03).14 The RISK-HIA (Register of Information and Knowledge about Swedish Heart Intensive Care Admissions) included 26,205 patients with STEMI, who received reperfusion therapy within 15 hours of symptom onset. Primary PCI was superior to prehospital thrombolysis or in-hospital thrombolysis with regard to 30-day mortality (mortality rates: 4.9%, 7.6%, and 11.4% respectively) or 1-year mortality (mortality rates: 7.6%, 10.3%, and 15.9%, respectively).37 Primary PCI was also associated with shorter hospital stay and less re-infarction. Moreover, for treatment delays greater than 2 hours, mortality reductions with prehospital thrombolysis tended to decrease, whereas the benefits with primary PCI persisted, regardless of the amount of delay.37 On the basis of these data, as well as the negative findings of the studies on early thrombolysis in the settings of the facilitated PCI strategy despite earlier restoration of flow in a considerable number of patients (see section on Facilitated PCI), it is unlikely that the occasionally propagated therapy of prehospital (in-ambulance) thrombolysis will be able to compete with a primary PCI strategy. In addition, prehospital thrombolysis carries the risk of exposing a sizeable proportion of misdiagnosed patients to the hazards of thrombolysis. Therefore, as long as no specifically designed studies have provided evidence in support of prehospital thrombolysis over routine PCI in patients calling for medical assistance within 2 to 3 hours from symptom onset, the application of prehospital thrombolysis strategy should be discouraged.
Recent studies have demonstrated the existence of an association between door-to-balloon interval and survival in patients with STEMI. A report from the National Registry of Myocardial Infarction (NRMI) data, which included a cohort of 29,222 patients with STEMI treated with PCI within 6 hours of presentation, longer door-to-balloon intervals were associated with increased in-hospital mortality.38 In-hospital mortality was 3%, 4.2%, 5.7%, and 7.4% for door-to-balloon intervals of 90 minutes or less, 91 to 120 minutes, 121 to 150 minutes, and more than 150 minutes, respectively (P < 0.001).38 In a report based on the Danish medical registries of patients with STEMI (N = 6209 patients undergoing primary PCI within 12 hours from symptom onset) showed an association and dependence of long-term (median 3.4 years) mortality on the time-to-treatment interval. Thus, for delays 0 to 60 minutes, 61 to 120 minutes, 121 to 180 minutes, and 181 to 360 minutes, long-term mortality was 15.4%, 23.3%, 28.1%, and 30.8%, respectively.39 In a recent report from the National Cardiovascular Data Registry, which included 43,801 patients with STEMI, median door-to-balloon time was 93 minutes with 57.9% of patients treated within 90 minutes. Longer door-to-balloon times were associated with a higher adjusted risk of in-hospital mortality, which increased in a continuous nonlinear fashion (door-to-balloon 30 minutes, mortality 3%; 60 minutes, 3.5%; 90 minutes, 4.3%; 120 minutes, 5.6%; 150 minutes 7%; 180 minutes 8.4%; P < 0.001).40 It appears that both a high-risk profile and a shorter presentation delay may accentuate this association.41 Despite these data, door-to-treatment times have not decreased significantly in recent years, so greater efforts should be made to improve this parameter.38 Door-to-balloon time is an important indicator of patient characteristics and of the experience of the institution providing the primary PCI.35,42 Comorbid conditions, absence of chest pain, delayed presentation after symptom onset, less specific ECG findings, and hospital presentation during off-hours were associated with longer total door-to-balloon times.35 Longer door-to-balloon times were also encountered in patients with older age, female gender, nonwhite race, and complex medical histories.43 Door-to-balloon delay also depended heavily on hospital-related characteristics. Thus, presentation at night and treatment at lower-volume facilities were strong independent predictors of longer door-to-balloon interval.43,44 Greater experience with primary PCI is associated with shorter door-to-balloon times and lower in-hospital mortality in patients with STEMI treated with primary PCI.42 Notably, primary PCI was found to be superior to thrombolysis irrespective of the door-to-balloon time that the treating institution was able to achieve.14 A further study demonstrated that a combination of shorter door-to-balloon time (<90 min) with a shorter symptom onset-to-door time (<4 hr) was associated with lowest longer term mortality.45 Other studies have also demonstrated that short door-to-balloon times (≤90 min) are associated with lower mortality in early presenters but not in late presenters.46
 Interhospital Transfer for Primary Percutaneous Coronary Intervention
Interhospital Transfer for Primary Percutaneous Coronary Intervention
Two factors—the lack of PCI facilities in the majority of hospitals that receive patients with STEMI and the proven superiority of primary PCI over thrombolysis—have led to the concept of emergency interhospital transfer for primary PCI instead of initial thrombolysis in the presenting hospital in patients with STEMI. Earlier randomized trials of on-site thrombolysis versus interhospital transfer plus PCI performed in the last 10 years or so have confirmed that transfer of patients for primary PCI is a better strategy than thrombolysis at the initial hospital. The results of these trials have been summarized in 2 meta-analyses. The first meta-analysis of six randomized trials performed before 2003, which included 3750 patients, showed that a strategy of patient transfer plus primary PCI was associated with a 42% reduction in the 30-day incidence of the combined endpoint of death, re-infarction, and stroke compared with a strategy of on-site thrombolysis.47 When the components of primary endpoints were considered separately, the strategy of transfer plus primary PCI was associated with a 68% reduction in re-infarction (P < 0.001), 56% reduction in stroke (P = 0.015), and 19% reduction in mortality (P = 0.08). Other quantitative reviews of studies that have involved patient transfer for PCI have suggested that for every 100 patients treated, primary PCI after interhospital transfer instead of on-site thrombolysis prevented 7 major adverse cardiac events defined as death, nonfatal re-infarction, or nonfatal stroke.48 Evidence suggests that patient transfer for PCI is also beneficial in patients with acute MI who receive full-dose thrombolytic therapy in a community hospital. In the Streptokinase In Acute Myocardial Infarction (SIAM-III) trial, patients presenting within 12 hours of acute MI were randomized to receive immediate stenting within 6 hours (n = 82) or delayed stenting at 2 weeks (n = 81) after full-dose reteplase. Immediate stenting was associated with a significant reduction of the combined endpoint (death, re-infarction, ischemic events, and target vessel revascularization) at 6 months (25.6% vs. 50.6%, P = 0.001).49 In keeping with this, recent studies provided further evidence of the benefits of transfer of patients for primary PCI compared with on-site thrombolysis. A recent study randomized 401 patients presenting to community hospitals to a strategy of on-site thrombolysis or to intravenous tirofiban and transport for primary PCI. The delay to reperfusion, defined as the interval from admission to start of thrombolysis or primary PCI, was 35 and 145 minutes, respectively, for the on-site thrombolysis and transport for PCI. The composite endpoint of death, re-infarction, or stroke was lower among patients assigned to the transport for primary PCI strategy at 30 days (8% vs. 15.5%, P = 0.019) and 1 year (11.4% vs. 21.5%, P = 0.006).50 A recent report of 850 patients with STEMI enrolled in the PRimary Angioplasty in patients transferred from General community hospitals to specialized PTCA Units with or without Emergency thrombolysis (PRAGUE)-2 trial showed that the 5-year composite endpoint of death, re-infarction, stroke, or revascularization was 40% in patients assigned to a strategy of transfer for primary PCI versus 53% in patients assigned to on-site thrombolysis in the presenting hospital (P < 0.001).51 A large registry that included 16,043 patients with STEMI who were treated with in-hospital thrombolysis, 3078 treated with pre-hospital thrombolysis, and 7084 patients treated with primary PCI, indicated that transfer for PCI is better than prehospital thrombolysis even in early presenters for whom the treatment is initiated within 2 hours.37 In aggregate, the results of the randomized trials and registries provide clear evidence in favor of the transfer of patients for primary PCI.
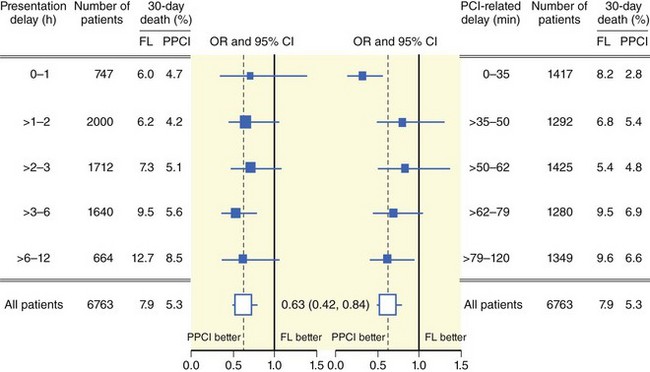
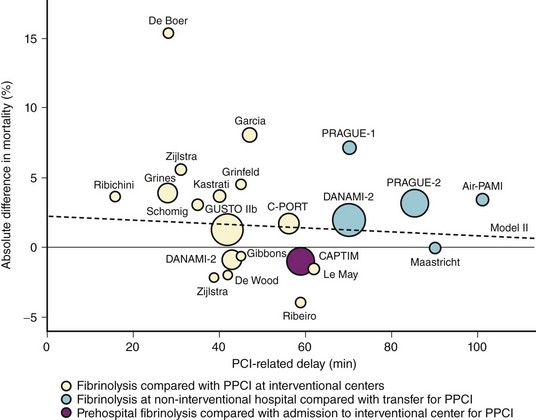
A strategy of patient transfer for primary PCI instead of on-site thrombolysis inevitably incurs additional delays imposed by transportation, logistics, and the organizational and technical aspects of PCI procedures. However, primary PCI has been found to be superior to thrombolysis despite these delays.7 PCI-related delay has become an integral part of treatment algorithms for patients with STEMI, although the evidence in support of this choice is poor and the assessment of this parameter is very difficult. No specific study has addressed this issue, and, in fact, the definition of the time point in PCI-related delay at which thrombolysis and primary PCI are at equipoise (and beyond which primary PCI might become inferior to thrombolysis) was based almost solely on a post hoc analysis of randomized clinical trials. A prior study that performed a regression analysis of mortality benefit from primary PCI or thrombolysis versus difference in time between application of these therapeutic options defined a PCI-related delay of 60 minutes as the time point beyond which primary PCI was inferior to thrombolysis.52 Although this analysis was later found to be flawed in several aspects, to a large extent, the 60-minute window for acceptable PCI-related delay is still endorsed in the recent ACC/AHA guidelines for the management of patients with STEMI.9,53 More recent studies have corrected and expanded the PCI-related delay at which the mortality rates of the two strategies are equal to 110 minutes, and 119 minutes (Fig. 18-11).53,54 In all these studies, the PCI-related delay was calculated on the basis of published summarized data and not on individual patient data. The most thorough time-based analysis that used individual patient data showed that primary PCI is superior to thrombolysis up to a PCI-related delay of 120 minutes.14 In this study, even in the group of patients presenting within 1 hour, mortality was lower with primary PCI (4.7% vs. 6%), indicating that even in early presenters with PCI-related delays of 60 minutes or less, there is no reason to prefer thrombolysis over primary PCI as the reperfusion strategy. The Vienna registry reported a comparable in-hospital mortality (8.1% vs. 8.2%) in patients treated with primary PCI versus thrombolysis at a mean PCI-related delay of 138 minutes.55 In 192,509 patients entered into the NRMI 2–4 registries, the mean PCI-related delay at which mortality benefits of primary PCI and thrombolysis were at equipoise was 114 minutes (95% confidence interval [CI] 96–132 minutes).56 Of note, the study showed that PCI-related delay was not static and varied considerably, depending on the risk characteristics of patients such as age, symptom duration, and infarct location. Thus, PCI-related delay varied from less than 1 hour for patients under 65 years of age with anterior infarction who presented within 2 hours to almost 3 hours for patients over 65 years of age with nonanterior infarction who presented later than 2 hours from symptom onset. Indeed, a recent regression analysis of 16 randomized trials of primary PCI versus thrombolysis further emphasized the impact of baseline risk on the PCI-related delay at which both therapies are at equipoise with regard to mortality.57 Another recent regression analysis, which included 27 trials with 4399 patients randomized to primary PCI and 4474 patients randomized to thrombolysis, found that the higher the risk of patients, the larger was the reduction in mortality achieved by primary PCI. It was calculated that for each 10-minute increase of PCI-related delay, there was a 0.75%, 0.45%, and 0% mortality benefit in high, medium, and low risk patients, respectively.58 Although these studies showed that longer delays reduce the survival benefits of primary PCI, such delays could be acceptable and even beneficial in high-risk STEMI patients. In essence, these studies advocate the use of flexible PCI-related delay according to the risk profile of STEMI patients. However, a longer PCI-related delay is often associated with less experience and inadequate infrastructure at the primary PCI center, which introduces an inevitable bias that is difficult to adjust for in this kind of analysis.42 Thus, assessment of the relationship between PCI-related time delay and outcome provides helpful information for the optimization of the primary PCI network but contributes little to the discussion about choosing the right reperfusion option for various settings. In an attempt to shorten PCI-related time delays, direct transportation of patients to hospitals that are capable of performing primary PCI rather than to the nearest hospital without PCI facility has also been suggested.59
In summary, prompt referral of patients with acute MI to centers with PCI facilities should be the primary objective of the first-contact emergency medical system. This is currently feasible for the large majority of patients with STEMI in the United States and should be attempted in the future for all patients with STEMI seeking medical aid. Nearly 80% of the adult population in the United States lives within 60 minutes of a hospital with PCI facility; even among those living closer to hospitals without PCI facility, almost three fourths would experience less than 30 minutes of additional delay in direct referral to a hospital with PCI facility.60 The development of regional systems of STEMI care is a matter of utmost importance for improving the treatment of patients with STEMI.22,23 Although not derived from specifically designed studies, a maximal 90- to 120-minute PCI-related delay seems to be reasonable. A flexible consideration of PCI-related delay, implying acceptance of longer delays for high-risk STEMI patients, seems also justified.
 Facilitated Percutaneous Coronary Intervention
Facilitated Percutaneous Coronary Intervention
Facilitated PCI refers to a deliberate strategy of administration of pharmacologic therapy aimed at restoring anterograde flow in the infarct-related artery before proceeding to definitive revascularization with PCI in patients with STEMI. It is conceived as an option for filling the time gap between patient presentation and performance of PCI. The pharmacologic regimen consists of drugs known for their ability to restore blood flow such as full-dose or half-dose thrombolysis or a combination of half-dose thrombolysis with GP IIb/IIIa receptor antagonists. As data about the ability of GP IIb/IIIa receptor antagonists to reopen the infarct-related artery are controversial, the isolated use of these drugs may or may not be part of the strategy of facilitated PCI. Two factors underpin the rationale behind the concept of facilitated PCI: (1) Ventricular function and prognosis have been found to be better in patients with STEMI who present at the time of primary PCI with spontaneous TIMI flow grade 2 and 3 compared with those who have a TIMI flow grade of 0 and 1 in the infarct-related artery; (2) a large proportion of patients with STEMI are unable to receive mechanical reperfusion without a certain amount of delay because of a variety of reasons. Facilitated PCI was hypothesized to offer a reduction in ischemia time, earlier reperfusion, higher TIMI flow rates in the infarct-related artery and facilitated guidewire or balloon passage, decreased clot burden, and lower incidence of distal embolization. The Bavarian Reperfusion Alternatives Evaluation (BRAVE) trial was the first randomized trial to evaluate the impact of facilitated PCI with reteplase plus abciximab on left ventricular infarct size estimated by single photon emission computed tomography (SPECT). Although the study reported a higher rate of pre-PCI TIMI flow grade 3 in the infarct-related artery in the facilitated PCI group, no reduction in infarct size was observed.61 These results were confirmed by several subsequent clinical trials on this issue. The Assessment of the Safety and Efficacy of a New Treatment Strategy for Acute Myocardial Infarction (ASSENT)-4 PCI study was a randomized trial of patients with STEMI presenting within 6 hours from symptom onset, who were scheduled to undergo PCI after an anticipated delay of 1 to 3 hours and were assigned to standard PCI (n = 838) or to PCI preceded by administration of full-dose tenecteplase (n = 829).62 All patients received aspirin and a bolus without an infusion of unfractionated heparin. The investigators of ASSENT-4 PCI planned to enroll 4000 patients, but the Data and Safety Monitoring Board recommended early cessation because of higher in-hospital mortality in the facilitated PCI group than in the group with standard PCI. The primary endpoint of ASSENT-4 PCI was death, congestive heart failure (CHF), or shock within 90 days from randomization. This occurred in 19% of patients in the facilitated PCI group and 13% in the standard PCI group (relative risk [RR] 1.39; 95% CI 1.11–1.74, P = 0.005). There were more in-hospital strokes (1.8% vs. 0%, P < 0.001) and higher incidence of ischemic complications such as re-infarction (6% vs. 4%; P = 0.03) and repeat target vessel revascularization (7% vs. 3%; P = 0.004) among patients treated with facilitated PCI than among those treated with standard PCI. The ASSENT-4 PCI trial concluded that a strategy of facilitated PCI consisting of full-dose thrombolysis (tenecteplase) plus concurrent anti-thrombotic therapy that preceded PCI by 1 to 3 hours was associated with a worse clinical outcome than a strategy of primary PCI alone; therefore, this strategy is not recommended.62 The meta-analysis of Keeley et al, which included 17 trials of patients with STEMI assigned to facilitated PCI (n = 2237) or primary PCI (n = 2267), showed that facilitated PCI was associated with significantly worse short-term outcomes (up to 42 days) than primary PCI alone: death (5% vs. 3%), nonfatal re-infarction (3% vs. 2%), urgent target vessel revascularization (4% vs. 1%), major bleeding (7% vs. 5%), hemorrhagic stroke (0.7% vs. 0.1%), and total stroke (1.1% vs. 0.3%).63 The increased rates of adverse events were observed mainly when thrombolytic therapy was used to facilitate PCI.63 The recently published FINESSE (Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events) study was another confirmation of the inefficacy and even detrimental effects of facilitated PCI in patients with STEMI.64 The study enrolled 2452 patients with STEMI presenting within the first 6 hours from symptom onset, who were randomly assigned to facilitated PCI with half-dose reteplase, abciximab versus abciximab alone, or conventional PCI with abciximab given in the catheterization laboratory (cath-lab). All patients received unfractionated heparin or enoxaparin before PCI and a 12-hour infusion of abciximab after PCI. The primary endpoint was the composite of death from all causes, ventricular fibrillation occurring more than 48 hours after randomization, cardiogenic shock, and CHF during the first 90 days after randomization. In the combination therapy–facilitated group, the abciximab-facilitated group, and the primary PCI group, the primary endpoint occurred in 9.8%, 10.5%, and 10.7% of the patients, respectively (P = 0.55, Fig. 18-12); 90-day mortality rates were 5.2%, 5.5%, and 4.5%, respectively (P = 0.49); early ST segment resolution occurred in 43.9%, 33.1%, and 31% (P = 0.003 for combination-facilitated PCI versus primary PCI, and P = 0.01 for combination-facilitated PCI versus abciximab-facilitated PCI). Overall, there was a graded increase in the rates of bleeding, intracranial hemorrhage, and transfusions in the PCI-facilitated groups.64 The evidence offered by the ASSENT-4 PCI trial, the meta-analysis by Keeley et al., and the FINESSE trial discourages the use of thrombolytics either at full dose or at half dose combined with GP IIb/IIIa inhibitors as pharmacologic facilitation of PCI.62–65
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree