The presentation of patients with suspected non ST-elevation acute coronary syndromes is quite diverse. Therefore, the diagnostic workup and choice of treatment may vary accordingly. Major issues regarding the evaluation are the likelihood of the diagnosis and the risk for adverse events. These factors should guide the choice of diagnostic test. Patients with increased risk for ischemic events and patients with recurrent ischemia are most likely to benefit from revascularization. In addition, when percutaneous coronary intervention is considered, evidence suggests that sufficient time should be allowed for pharmacologic stabilization, reducing the possibility of periprocedurally inflicted myocardial infarction. However, postponement of intervention may lead to an increase of new spontaneous events, and high-risk patients should apply for revascularization soon after pharmacologic stabilization. The extent of revascularization performed by percutaneous coronary intervention depends predominantly on patient characteristics and anatomy but should be limited to flow-obstructive lesions. In conclusion, patients presenting with non–ST elevation acute coronary syndromes constitute a very diverse population; diagnostic workup, treatment, and the timing of a possible intervention should be tailored individually.
Patients with chest pain represent a large and increasing proportion of all acute medical presentations worldwide. Of all those presenting for evaluation, only a minority have acute coronary syndromes (ACS). Distinguishing which patients have ACS remains a diagnostic challenge. The principal pathophysiologic mechanism of ACS is myocardial underperfusion, which is caused by atherosclerotic plaque rupture or erosion, with different degrees of superimposed thrombus. Electrocardiography provides the initial classification. Patients are divided into those with persistent ST-segment elevation and those without persistent ST-segment elevation or non ST-elevation ACS (NSTEACS). In this review, we discusses the diagnostic challenges when NSTEACS are suspected. In addition, we address the role of risk stratification in relation to the choice of treatment strategy. When an invasive approach is preferred, an important issue is the timing of the intervention. The available evidence on this topic is discussed in detail. We conclude with the evidence regarding the type and extent of revascularization in patients with multivessel disease.
Diagnostics and Risk Assessment
In patients presenting with suspected NSTEACS, 2 major issues must be addressed. The first challenge is to confirm the diagnosis. Guidelines recommend the use of elementary tools, such as symptoms, risk profile for coronary artery disease, electrocardiography, and biomarkers, to estimate the likelihood of disease. In addition, echocardiography in the acute phase can be used to clarify the diagnosis. However, the diagnosis sometimes remains uncertain. In these cases, the clinical probability of ACS should be assessed. Although American College of Cardiology (ACC) and American Heart Association (AHA) as well as European Society of Cardiology (ESC) guidelines do not provide guidance on this topic, the next diagnostic test of choice should depend on the likelihood of disease. In Figure 1 , an algorithm is proposed in which the preferred performance of a diagnostic test is related to the estimated probability of NSTEACS. In case of low clinical probability, patients are to be discharged safely, so a diagnostic test should be used with high sensitivity and high negative predictive value. Ischemia testing such as exercise testing with or without an imaging modality is frequently used in the subacute setting. However, such tests are most useful in patients with intermediate probability of ACS. In our opinion, poorly performing tests, such as treadmill or bicycle exercise tests, should be restricted to prognostic purposes only. Despite being not recommended by current ESC guidelines, computed tomographic angiography (CTA) is at present the most accurate noninvasive test to rule out coronary artery disease. New sophisticated scan protocols, using prospective electrocardiographically gated triggering, substantially reduce radiation exposure (effective dose value approximately 3 mSv), without reducing image quality. Extracardiac findings such as pulmonary tumors, pulmonary embolism, and aortic dissection can also be detected. In selected patients with acute chest pain, the diagnostic accuracy of CTA is excellent. In addition, this approach is more cost effective and less time-consuming.
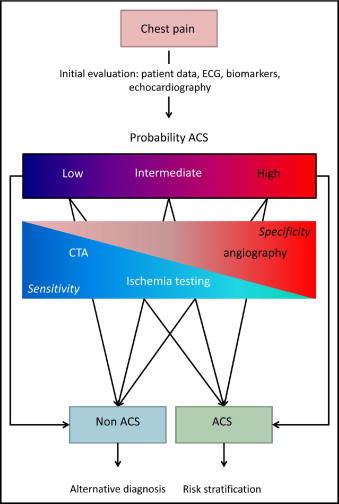
In case of a high probability of ACS, patients should be admitted to the hospital for clinical follow-up and treatment. In these patients, false-positive results are more likely to occur. Accordingly, the diagnosis of ACS should be waived only on the basis of tests with high sensitivity and specificity, invasive coronary angiography currently being the gold standard. In this population, coronary angiography is able to exclude coronary artery disease reliably. This should be strived for, because even in the presence of electrocardiographic changes and troponin increase, about a fifth of the patients suspected of high-risk NSTEACS show no significant lesions on coronary angiography. These patients generally are at low risk and should be evaluated for alternative pathologies.
Because of the absence of validated scoring systems to estimate the probability of NSTEACS in patients with chest pain, there is limited information on the distribution of the eventual diagnoses across the various levels of suspicion of ACS. A small trial by Goldstein et al evaluated the use of CTA in about 200 patients with chest pain and low probability of ACS. The mean Thrombolysis In Myocardial Infarction (TIMI) risk score was 1.2. The number of patients diagnosed with NSTEACS was about 10%, and the remainder had noncardiac chest pain. The percentage of patients who underwent percutaneous coronary intervention (PCI) was 4%, and the percentage requiring coronary artery bypass grafting (CABG) was 2%. In the Optimal Timing of PCI in Unstable Angina (OPTIMA) trial, about 250 patients with suspected intermediate- to high-risk NSTEACS underwent acute coronary angiography. The mean TIMI risk score was 3.8. Of these, 78% were diagnosed with NSTEACS, and the remainder had noncardiac chest pain. Of all patients, 55% were treated with PCI, and 10% underwent CABG.
The second issue to be addressed in patients with suspected NSTEACS involves risk assessment. Patients with NSTEACS represent a prognostically heterogenous group. Therefore, risk stratification plays a central role in evaluation and management. For this purpose, multiple scoring models have been developed, with the Global Registry of Acute Coronary Events (GRACE) and TIMI risk scores being the most widely used. The 2 models show a strong relation between indicators of the likelihood of NSTEACS and prognosis. The GRACE risk tool was developed on the basis of data from a large multinational cohort study (GRACE) and validated in subsequent GRACE and Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) IIb cohorts. Recently, the GRACE score was prospectively revalidated in a large contemporary cohort. The TIMI score was developed using data from the TIMI 11B trial and prospectively validated in several cohorts, including that of the Treat Angina With Aggrastat and Determine Cost of Therapy With an Invasive or Conservative Strategy (TACTICS)–TIMI 18 trial. The GRACE score estimates the risk for death up to 6 months, and the TIMI risk score addresses the 14-day risk for death, recurrent myocardial infarction (MI) or urgent revascularization. This risk estimation, together with individual patient characteristics, should further guide treatment strategy.
Indications for Urgent Revascularization
A subset of patients with NSTEACS are considered to have such an increased mortality risk that immediate revascularization is recommended. These include cardiogenic shock, severe left ventricular dysfunction, suspected left main stem disease, recurrent or refractory ischemia at rest despite intensive pharmacologic treatment, mechanical complications such as acute mitral regurgitation, and sustained ventricular tachycardia. This recommendation is based on a single study that suggested better outcomes with revascularization in patients presenting with cardiogenic shock. However, most patients can be medically stabilized. These patients should be evaluated for an invasive approach.
Indications for Urgent Revascularization
A subset of patients with NSTEACS are considered to have such an increased mortality risk that immediate revascularization is recommended. These include cardiogenic shock, severe left ventricular dysfunction, suspected left main stem disease, recurrent or refractory ischemia at rest despite intensive pharmacologic treatment, mechanical complications such as acute mitral regurgitation, and sustained ventricular tachycardia. This recommendation is based on a single study that suggested better outcomes with revascularization in patients presenting with cardiogenic shock. However, most patients can be medically stabilized. These patients should be evaluated for an invasive approach.
Routine Invasive Versus Selective Invasive Therapy
In the past 2 decades, multiple trials have evaluated different clinical strategies regarding coronary angiography and subsequent revascularization of clinically stabilized patients with NSTEACS. Two general approaches have emerged, the first being the “early invasive” or “routine invasive” strategy, involving routine early coronary angiography followed by revascularization when appropriate. The second is the “conservative” or “selective invasive” approach, with initial pharmacologic management and coronary angiography followed by revascularization for recurrent ischemia only. This new ischemia may either be spontaneous or provoked by noninvasive stress testing. Currently, AHA and ACC as well as ESC guidelines support routine invasive management in intermediate- to high-risk patients with NSTEACS.
Four large randomized controlled trials have dominated the debate on the routine performance of invasive diagnostics in NSTEACS. The results, unfortunately, were quite diverse ( Table 1 ). In 1999, the Fragmin and Fast Revascularisation During Instability in Coronary Artery Disease (FRISC) II trial showed a significant reduction in the combined end point of death and MI with the routine invasive approach. The observed difference was driven mainly by an excess in MI in the selective invasive group. The TACTICS–TIMI 18 trial, published in 2001, showed similar results: a decrease in MI but no significant mortality benefit. In 2003, the Randomized Intervention Trial of Unstable Angina (RITA) 3 trial failed to show any benefit for death or MI. Ultimately, in 2005, the Invasive Versus Conservative Treatment in Unstable Coronary Syndromes (ICTUS) trial was published. This study, with optimal medical treatment in both arms, showed an increased MI risk in the routine invasive arm, with no difference in mortality.
| Trial | Time Frame Patient Inclusion | Timing of Catheterization in the Routine Invasive Approach | RI | SI | Primary Trial Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Revascularization | % PCI | % Revascularization | % PCI | MI | Mortality | |||||
| RI | SI | RI | SI | |||||||
| FRISC II | 1996–1998 (6-month FU) | <7 days | 77% | 42% | 37% | 18% | 94/1,207 ⁎ (7.8%) | 124/1,226 (10.1%) | 23/1,207 (1.9%) | 36/1,226 (2.9%) |
| TACTICS–TIMI 18 | 1997–1999 (6-month FU) | 4–48 hours | 64% | 42% | 35% | 29% | 53/1,114 † (4.8%) | 76/1,106 (6.9%) | 37/114 (3.3%) | 39/1,106 (3.5%) |
| RITA 3 | 1997–2001 (1-year FU) | <72 hours | 57% | 36% | 28% | 16% | 34/895 (4%) | 44/915 (5%) | 41/895 (4.5%) | 36/915 (4%) |
| ICTUS | 2001–2003 (1-year FU) | 24–48 hours | 79% | 61% | 54% | 40% | 90/604 ‡ (15%) | 59/596 (10%) | 15/604 (2.5%) | 15/596 (2.5%) |
Interpretation of the study results is difficult because of important differences in method. Foremost, when the studies are compared, there appears to be a marked variation in the intensity of revascularization between study arms ( Table 1 ). The conservative arm of the ICTUS trial showed a revascularization rate similar to the routine invasive arm in RITA 3. Also, the definition of MI differed between the trials. The low biomarker threshold used in the ICTUS trial may partly explain the higher number of MIs in patients requiring PCI.
The improved use of anticoagulants, dual-antiplatelet therapy, statins, and angiotensin-converting enzyme inhibitors may also be part of the assumed demise of the routine invasive treatment benefit. This is most clear for the use of statins. In the FRISC II and TACTICS–TIMI 18 16 trials, approximately 1/2 the patients received statins at discharge. In RITA 3, this had already increased to 70%, whereas the ICTUS trial provided high-dose statin treatment to 92% of patients. Although less sharp, the use angiotensin-converting enzyme inhibitors showed similar patterns. On the basis of the ICTUS trial, the current AHA and ACC guidelines acknowledge the option of a selective invasive strategy with aggressive medical treatment.
It may not be surprising that long-term (5-year) follow-up of the aforementioned trials showed discordant results. Remarkably, the initial negative RITA 3 trial suggested a marked 5-year benefit in the routine invasive group regarding death and MI (odds ratio 0.78, 95% confidence interval 0.61 to 0.99, p = 0.04). Indeed, a recent meta-analysis based on individual 5-year follow-up patient data from FRISC II, RITA 3, and ICTUS showed a reduction in MI using the routine invasive strategy.
The relation between treatment effect and patient risk has been evaluated in subanalyses of several trials. Regardless of the risk score used, there appeared to be a consistent treatment benefit for the invasive approach in high-risk patients compared to low-risk patients. The FRISC II and TACTICS–TIMI 18 trials as well as the 5-year follow-up of RITA 3 showed the greatest benefit of the routine invasive approach in high-risk patients. This resulted in a wide acceptance of the routine invasive approach in this subpopulation. The clinical application of the aforementioned TIMI and GRACE risk scores has been evaluated extensively. Remarkably, recent data from the GRACE registry suggest the presence of an inverse relation between patient risk and the rate of PCI. In daily practice, angiographic findings and referral practice may more substantially influence the decision to proceed to PCI than patients’ risk status.
In conclusion, the different outcomes in the large trials evaluating the invasive approach in NSTEACS mainly reflect the changes in study protocols and in pharmacologic treatment. For clinical practice, it seems reasonable to consider a liberal selective invasive approach equivalent to a temperate routine invasive approach. The patients with the highest risks for adverse outcomes are thought to derive the greatest benefit from invasive evaluation and revascularization. However, because clinical judgment on risk estimation appears to be challenging, the use of systematic and accurate risk stratification methods seems important.
Timing of Percutaneous Coronary Intervention
In the past few years, several studies have evaluated the influence of the timing of intervention in patients with NSTEACS. Once again, comparison of data and interpretation of the results are difficult, mainly because of methodologic differences among the studies ( Table 2 ). Current AHA and ACC as well as ESC guidelines do not give specific recommendations on this topic.
| Trial | Time Frame Patient Inclusion | Early strategy | Delayed strategy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median Time to Catheterization (hours) | % PCI | Median Time to PCI (hours) | Median Time to catheterization (hours) | % PCI | Median Time to PCI (hours) | Trial Outcome at 1 Month | |||||
| MI | Mortality | ||||||||||
| Early | Delayed | Early | Delayed | ||||||||
| ISAR COOL | 2000–2002 | 2.4 | 64% | NA | 86 | 70% | NA | 12/203 (5.9%) | 21/207 (10.1%) | 3/207 (1.4%) | 0/203 (0.2%) |
| TIMACS | 2003–2008 | 14 | 60% | 16 | 50 | 55% | 52 | 57/1,593 (3.6%) | 59/1,438 (4.1%) | 46/1,593 (2.9%) | 47/1,438 (3.3%) |
| OPTIMA | 2004–2007 | 2 | 100% | 2.5 | 1.8 | 99% | 27 | 44/73 ⁎ (60.3%) | 26/69 (37.7%) | 0/73 (0%) | 0/69 (0%) |
| ABOARD | 2006–2008 | 1.1 | 80% | NA | 20.5 | 70% | NA | 16/175 (9.1%) | 8/177 (4.5%) | 5/175 (2.9%) | 2/177 (1.1%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


