Percutaneous Coronary Intervention
Despite tremendous advances in the care of patients with heart disease seen over the past 30 years, coronary artery disease remains the leading cause of mortality and morbidity in the world.1 With an international population that is increasingly affected by hypertension, diabetes, and obesity, the incidence of coronary artery disease will continue to affect large proportions of the world’s population.2 Advances in the medical treatment of patients with coronary artery disease have improved outcomes over this period of time; however, the use of revascularization to prevent mortality, improve anginal symptoms, and increase exercise capacity remains a fundamental component in management in patients with ischemic heart disease.3–5
Revascularization strategies were initially limited to surgical procedures such as coronary artery bypass grafting (CABG). This paradigm began to shift in 1977 when Andreas Gruentzig performed the first percutaneous transluminal coronary angioplasty (PTCA) that successfully increased coronary blood flow previously limited by coronary atherosclerosis. Initial attempts at percutaneous revascularization were hindered by high rates of acute closure and restenosis of the treated lesions.6 With the development of more potent antiplatelet agents such as thienopyridines and the increased use of coronary stents (percutaneous coronary intervention [PCI]), these limitations have been reduced such that PCI is now the predominant form of coronary revascularization in the United States.7
As such, PCI has been the focus of some of the most important clinical trials in cardiology that have shaped clinical practice and defined the algorithms established in the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.3–5 The current format of the cardiovascular board exam focuses approximately 25% of the content on the management of patients with coronary artery disease and acute myocardial infarction (MI). Revascularization strategies are an important component in the management of these problems. The constant evolution in both the management of patients with ischemic heart disease and the use of PCI makes it challenging to present from the perspective of the Cardiology Board exam, which is based mostly on the most current ACC/AHA guidelines, and thus lags current practice by a few years. In this chapter, we focus briefly on the techniques and complications of PCI while devoting increased attention to the ACC/AHA guidelines and major clinical trials evaluating the role of PCI in the management of patients with ischemic heart disease.
INDICATIONS AND APPROPRIATENESS OF PCI
ST-Elevation Myocardial Infarction
It is now well established that ST-elevation myocardial infarction (STEMI) occurs when an atherosclerotic plaque ruptures or erodes leading to thrombosis in an epicardial coronary artery. Initial attempts at restoring coronary flow utilized medications with fibrinolytic capabilities designed to breakdown the coronary thrombosis. These therapies, tested in numerous clinical trials (GUSTO, GISSI, ISIS-2, FTT), clearly showed that patients treated with reperfusion therapy had improved survival as compared to those patients treated with conservative management.8–11 Fibrinolytic therapy was hindered by its relatively high rates of intracranial hemorrhage leading to the search for improved revascularization techniques.12
Initial attempts to treat STEMI with percutaneous therapies were limited to balloon angioplasty (PTCA). Studies comparing primary PTCA to fibrinolytic therapy demonstrated less recurrent ischemia, reinfarction, reocclusion, and strokes in patients treated with primary PTCA. While the long-term mortality benefit of primary PTCA was not consistent in the various studies (GUSTO-2B, PAMI), a metaanalysis by Keeley et al. showed a 23% relative reduction in mortality (7% in PTCA vs. 9% in fibrinolytic; p = 0.0002) for PTCA compared to thrombolysis.13,14 Since these trials were conducted, additional therapies such as coronary stenting (which prevents acute vessel closure) and aspiration thrombectomy (removes clot from the coronary artery) have been developed and found to be beneficial in patients with STEMI.15 Studies have shown that the use of coronary stents in STEMI is more effective than PTCA alone in increasing postintervention lumen size and decreasing the risk of acute vessel closure, subsequent ischemic events, and repeat target vessels revascularization (GRAMI, FRESCO, stent-PAMI).16–18 There have been no studies demonstrating a survival benefit for stents compared to PTCA alone.
The superiority of PCI as compared to fibrinolytic therapy is reflected in the current ACC/AHA guidelines that support the use of primary PCI in patients with STEMI or chest pain and a new left bundle branch block in situations where it can be performed in a timely manner (door to balloon time of <90 minutes). The timing of symptom onset is an important component of the decision to pursue percutaneous revascularization. For patients with symptom onset within 12 hours of presentation, primary PCI is given a Class I indication. Patients with the onset of symptoms within 12 to 24 hours who have severe congestive heart failure, hemodynamic/electrical instability, or evidence of persistent ischemia (i.e., continued chest pain) are also candidates for percutaneous revascularization (Class IIa recommendation). Asymptomatic patients who present more than 12 hours after the onset of symptoms should not be treated with percutaneous revascularization (Class III recommendation) (Table 47.1).
TABLE
47.1 Recommendations for the Use of PCI in Patients with STEMI
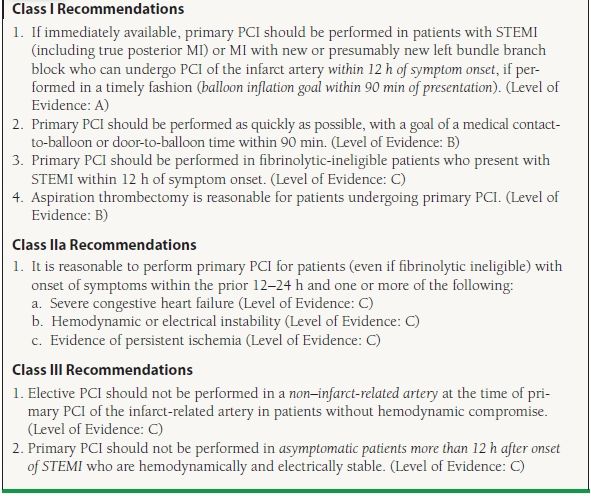
Modified from the ACC/AHA guidelines: King SB III, Smith SC Jr, Hirshfeld JW Jr, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51:172–209; Smith SC Jr, Feldman TE, Hirshfeld JW Jr, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). Circulation. 2006;113:156–175; Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241.
PCI in Cardiogenic Shock
Approximately 5% of patients with STEMI have a significant decrease in cardiac output resulting in end-organ malperfusion. This condition, known as cardiogenic shock, is defined clinically by the presence of a systolic blood pressure <90 mm Hg lasting for more than 30 minutes (in the absence of hypovolemia) or vasopressors required to achieve a systolic blood pressure ≥90 mm Hg combined with a reduced cardiac index (1.8 L/min/m2) and evidence of an elevated left ventricular filling pressure (i.e., pulmonary capillary wedge pressure >18 mm Hg).19 Patients who develop cardiogenic shock are at increased risk of death with mortality rates ranging from 30% to 50%.20 The SHOCK trial was a landmark trial that serves as the basis for the current ACC/AHA recommendations for the treatment of patients in cardiogenic shock. This trial showed that revascularization performed within 36 hours from symptom onset was superior to initial medical stabilization with a lower mortality rates at 6 months (50% with PTCA vs. 63% with initial medical therapy, p = 0.027).21
Subset analysis from the SHOCK trial showed that patients ≥75 years old did not have the same benefit from revascularization raising questions as to whether patient revascularization should be pursued in the elderly. While subsequent observational and post hoc analysis have provided support for the use of revascularization in the elderly, this ambivalence is reflected in the current ACC/AHA guidelines (Table 47.2).22
TABLE
47.2 Recommendations for the Use of PCI in Patients with Cardiogenic Shock
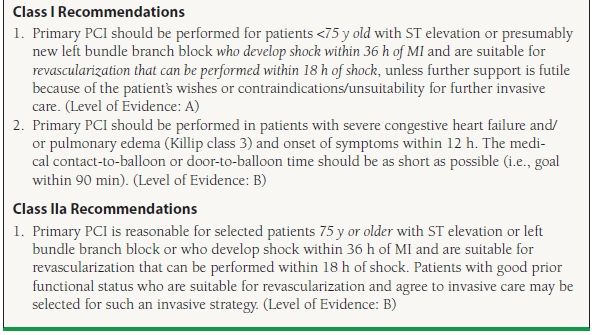
Modified from the ACC/AHA guidelines: King SB III, Smith SC Jr, Hirshfeld JW Jr, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51:172–209; Smith SC Jr, Feldman TE, Hirshfeld JW Jr, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). Circulation. 2006;113:156–175; Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241.
Role of PCI in Patients Treated with Fibrinolytics
Not all hospitals are equipped with catheterization laboratories capable of performing emergent PCI in the setting of STEMI. As a result, some patients with STEMI may be treated with fibrinolytic therapy if transfer to a PCI hospital cannot be performed in a timely manner. Attempts to combine lower dose fibrinolytic therapies with PCI have largely been unsuccessful in improving outcomes. The FINESSE trial (Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events) randomized patients with STEMI to facilitated PCI (half-dose fibrinolytics + abciximab), abciximab-facilitated PCI, or primary PCI.23 At 90 days, there was no difference in the primary endpoint (composite of death from all causes, ventricular fibrillation occurring more than 48 hours after randomization, cardiogenic shock, and congestive heart failure during the first 90 days after randomization). Based on this study, facilitated PCI (fibrinolytics as an intentional prelude to primary PCI) should not be performed.
The lack of benefit for facilitated PCI does not imply that PCI does not have a role in the management of patients treated with fibrinolytic therapy. Up to 25% of patients treated with fibrinolytics will not achieve coronary reperfusion.24 Identifying this subset is important since patients with a patent infarct–related artery have improved clinical outcomes as compared to patients in which reperfusion is not successful. Continued chest pain and persistent ST elevations are typically considered markers of failed reperfusion; however, prior studies have found that approximately half of patients with persistent chest pain and ST elevations will actually have a patent infarct artery.24 Despite this limitation, persistent ST elevation of >50% remains an indication for rescue PCI (PCI performed after failed fibrinolysis) due to the large benefit of rescue PCI shown in the REACT trial. The REACT trial randomized patients who did not have a >50% reduction in ST elevation to conservative therapy, repeat thrombolysis, or rescue PCI. At 6 months, patients treated with rescue PCI were significantly more likely to be free from death, reinfarction, stroke, or severe heart failure (adjusted hazard ratios of 0.43; p = 0.001 for rescue PCI vs. repeated thrombolysis).25 In addition to the ECG requirement, ACC/AHA guidelines also support immediate PCI following the administration of fibrinolytic therapy for patients with recurrent MI (Class I indication), moderate or severe ischemia (Class I indication), cardiogenic shock/hemodynamic instability (Class I indication), LVEF <40% (Class IIa indication), and serious ventricular arrhythmias (Class IIa indication) (Table 47.3).
TABLE
47.3 Recommendations for PCI After Failed Fibrinolysis (Rescue PCI)
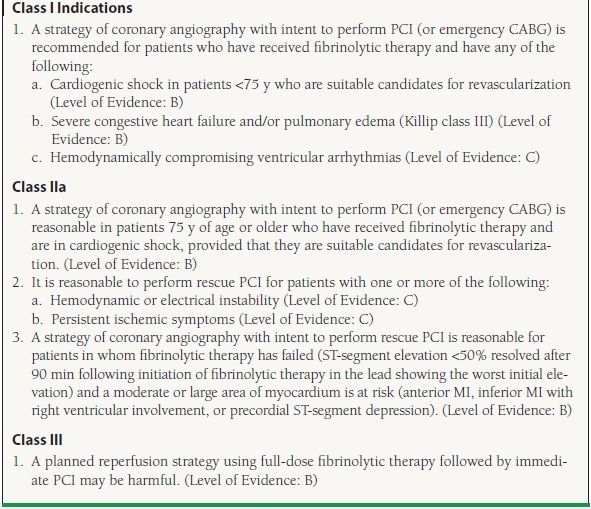
Modified from the ACC/AHA guidelines: King SB III, Smith SC Jr, Hirshfeld JW Jr, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51:172–209; Smith SC Jr, Feldman TE, Hirshfeld JW Jr, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). Circulation. 2006;113:156–175; Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241.
Patients should be transferred to a hospital with PCI capabilities after the administration of fibrinolytics due to the numerous potential indications for PCI. In patients with successful fibrinolysis who are asymptomatic, earlier trials that predated the routine use of clopidogrel and GP IIb/IIIa inhibitors showed no evidence that immediate PCI of the infarct-related artery provided any further reduction in death, reinfarction, or myocardial salvage (SWIFT and TIMI II). Other trials such as SIAM III, GARCIA-1, and CAPITAL-AMI showed significant reduction in mortality and ischemic events in those who underwent immediate PCI after successful fibrinolysis. A larger, definitive trial has also provided evidence of benefit. The TRANSFER-AMI study randomized patients to either standard treatment (including rescue PCI) or immediate transfer to another hospital for PCI within 6 hours after fibrinolysis.26 At 30 days, there was a significant decrease in the number of patients with death, reinfarction, recurrent ischemia, new or worsening congestive heart tailure, or cardiogenic shock (relative risk with early PCI, 0.64; p = 0.004).This has been further clarified with results of a meta-analysis by D’Souza et al., which showed that an early invasive PCI strategy following administration of fibrinolytic therapy results in a 53% reduction (odds ratio 0.47 [95% confidence interval (CI), 0.32-0.68, p <0.0001]) in a combined endpoint of 30-day mortality, reinfarction, and ischemia. The majority of the difference was in a significant reduction in both reinfarction and recurrent ischemia that 30-day mortality and major bleeding rates between strategies were not significantly different.27 While not yet reflected in the ACC/AHA guidelines, many providers now advocate routine angiography following the administration of fibrinolytic therapy; however, the optimal timing of PCI following the administration of fibrinolytic therapy remains unknown (Table 47.4).
TABLE
47.4 Recommendations for PCI After Successful Fibrinolysis or for Patients Not Undergoing Primary Reperfusion

Modified from the ACC/AHA guidelines: King SB III, Smith SC Jr, Hirshfeld JW Jr, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51:172–209; Smith SC Jr, Feldman TE, Hirshfeld JW Jr, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). Circulation. 2006;113:156–175; Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241.
Unstable Angina/Non–ST-Elevation Myocardial Infarction
The proportion of patients with high-risk unstable angina or non–ST-elevation acute coronary syndrome (NSTEMI) is increasing in the United States.28 Current ACC/AHA guidelines assume that patients with UA/NSTEMI are already on intensive medical therapy including aspirin, thienopyridine, antithrombotic (heparin, bivalirudin, etc.) beta-blockers, and statins. The role of revascularization in this population has been the focus of much attention and numerous clinical trials, including TIMI-IIIB, VANQWISH, FRISC II, TACTICS TIMI-18, RITA-3, and ICTUS. While there has been differences in the conclusions drawn from these studies, the bulk of the trials and a large meta-analysis support the use of an early invasive revascularization strategy (performance of diagnostic angiography with intent to perform revascularization within 48 hours of presentation).29 In patients with refractory angina or hemodynamic/electrical instability, revascularization should be performed in a semiemergent manner.30
While the early invasive strategy is preferred for patients who are suitable candidates for revascularization, the largest benefit comes from revascularization in patients considered to be high risk. Numerous risk prediction models, including the GRACE and PURSUIT risk scores, have been developed in order to guide the decision to refer to revascularization. The most well validated and widely used of these risk scores is the TIMI Risk Score that assigns patients one point for each of the following risk factors: age >65, known coronary artery disease (lesion >50%), three of more risk factors of coronary artery disease (tobacco abuse, family history, diabetes, hypertension, hyperlipidemia), aspirin use within 7 days, positive cardiac biomarkers, ST changes of >1 mm, and two or more episodes of chest pain within the past 24 hours. Patients with greater than two risk factors derive benefit from an early invasive strategy31 (Table 47.5).
TABLE
47.5 Risk Stratification in UA/NSTEMI
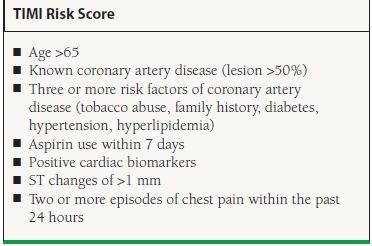
Adapted from Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842.
In patients who are stable, an initial conservative strategy may be considered even in high-risk patients if preferred by either patients or their physicians. While the guidelines support early catheterization for patients, this does not imply that all patients with UA/NSTEMI warrant revascularization. Revascularization is not indicated in patients with—one- to two-vessel CAD with either no symptoms or symptoms that are unlikely to be due to myocardial ischemia. In addition, revascularization is contraindicated in patients without high risk features who have only a small area of at-risk myocardium, lesions that have a low likelihood of revascularization, high risk of procedure-related morbidity/mortality, and stenosis of <50% or patients with left main CAD who are candidates for CABG (Table 47.6).
TABLE
47.6 Recommendations for PCI in Patients with UA/NSTEMI
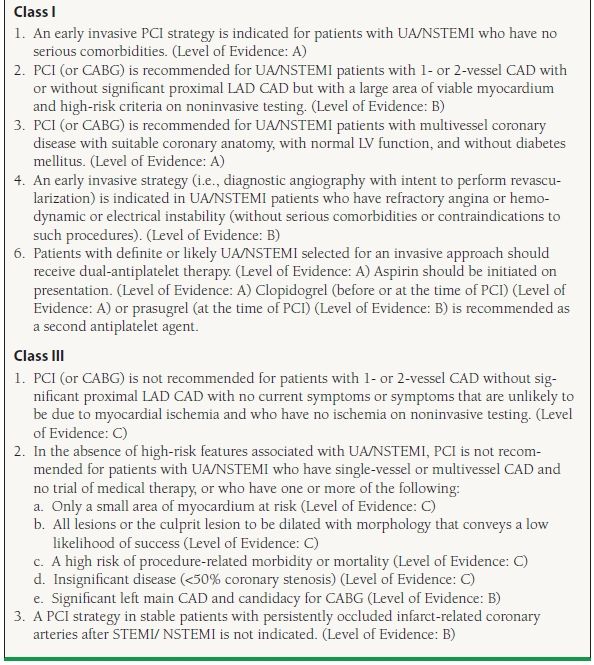
Modified from the ACC/AHA guidelines: King SB III, Smith SC Jr, Hirshfeld JW Jr, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51:172–209; Smith SC Jr, Feldman TE, Hirshfeld JW Jr, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). Circulation. 2006;113:156–175; Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241.
Stable Angina
Percutaneous revascularization was first utilized in patients with severe, chronic angina. In the current era, symptom relief from chronic stable angina remains the most frequent indication for revascularization. The decision to pursue medical therapy or revascularization is a highly individualized decision based on patient characteristics, symptoms, lifestyle, and preferences. The severity of angina is typically classified on the Canadian Cardiovascular Society Functional Classification of Angina Pectoris scale32 (Table 47.7).
TABLE
47.7 Canadian Cardiovascular Society Angina Pectoris Scale

From Campeau L. Letter: grading of angina pectoris. Circulation. 1976;54:522–523, with permission from Wolters Kluwer Health.
There is a significant amount of clinical trial data comparing outcomes in patients treated with medical therapy versus revascularization. Numerous clinical trials and observational studies have been conducted in an attempt to understand the patient populations most likely to benefit from PCI. The RITA-II trial (Second Randomized Intervention Treatment of Angina) randomized 1,018 patients with angina to either PTCA (only 8% received stents) or medical therapy.33 After 2.7 years, death or definite MI occurred in 6.3% of patients treated with PCI, whereas these endpoints occurred in 3.3% of patients with medical care (p = 0.02). The difference between the two groups was primarily due to seven periprocedural nonfatal MIs in the revascularization group (which are of questionable significance). Revascularization did result in significant improvement in angina. There was a 16.5% absolute excess of moderate/severe angina in the patients treated with medical therapy. In addition, patients treated with revascularization have longer exercise times on the Bruce protocol. By 7 years, the incidence of death or MI was comparable in both groups.34
The ACIP trial (Asymptomatic Cardiac Ischemia Pilot) evaluated a slightly different population.35 Five hundred and fifty-eight patients with evidence of ischemia who underwent angiography and were found to have coronary artery disease amenable to revascularization were randomized with angina-guided medical therapy, ischemia-guided medical therapy, or revascularization with CABG or PTCA. After 2 years, the total mortality was 6.6% in the angina-guided group, 4.4% in the ischemia-guided group, and 1.1% in those receiving revascularization. These data suggested a benefit from initial revascularization compared to medical therapy in patients with demonstrable ischemia.
The most recent trial of medical therapy versus revascularization trial in patients with stable coronary artery disease was the COURAGE study (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation).36 This trial randomized 2,287 patients with coronary artery disease and evidence of ischemia to either PCI with optimal medical therapy (PCI group) or initial optimal medical therapy. Approximately one out of every three patients initially treated with medical therapy had a revascularization at some point during the follow-up period. After a median follow-up of 4.6 years, there was no difference in either all-cause mortality or nonfatal MI between the two groups. Relief from angina was significantly improved in patients treated with revascularization plus optimal medical therapy. This difference was attenuated over time possibly due to the significant number of patients in the initial medical therapy arm who eventually underwent revascularization. On the basis of this study, patients with stable angina can be treated initially with a trial of medical therapy with revascularization reserved for those patients with persistent angina.
It is possible that the use of functional assessment of coronary artery stenosis using either fractional flow reserve or stress imaging may provide an appropriate way of risk stratifying those patients most likely to benefit from revascularization instead of initial medical management. For example, a substudy of the COURAGE trial showed that patients with a >5% reduction in the amount of ischemia had improved outcomes, and PCI was more effective than medical therapy in reducing myocardial ischemia.37 This provides support for the use of ischemia-guided PCI; however, it remains unclear whether reduction of ischemia with PCI will improve clinical outcomes in patients with stable coronary artery disease.
The continuing improvements in outcomes with revascularization and the individuality of each patient’s symptoms and coronary anatomy make definitive conclusions about generalized patient populations challenging. It is apparent that patients with chronic stable angina have more effective relief of angina with percutaneous revascularization; however, it is likely that there is no difference between the two strategies in regard to the risk of MI, death, or need for future PCI and/or CABG. Patients with more severe angina and demonstrable ischemia on stress testing have a greater benefit in regard to relief of angina, while the effect on mortality remains debatable. As a result, a strategy of initial medical management in this population is both safe and effective.
Appropriateness criteria have been developed by the ACC/AHA to help guide the decision on which patients are likely to have the greatest benefit from revascularization.38 These criteria were developed largely from expert opinions based on the limited data available and the clinical experience of those involved in formulating the guidelines. For the purposes of the boards, these criteria do not need to be committed to memory but are a useful reference for making decisions regarding the decision to pursue revascularization. Factors considered in the development of the appropriateness criteria for revascularization include the location and extent of the coronary disease, severity of symptoms, extent of ischemia on stress testing, and use of medical therapy. Those patients with increased severity of coronary artery disease, larger area of ischemia, and more severe symptoms are those patients most likely to benefit from revascularization therapy. In contrast, those patients with minimal symptoms, small areas of ischemia, and less extensive coronary artery disease are those who are most likely to benefit from medical therapy with aspirin, antianginal medication (nitrates, beta-blockers, calcium channel blockers), and statins. Revascularization is also often considered in patients with ischemic heart disease prior to planned surgery. There is no evidence that prophylactic revascularization improves outcomes as compared to medical therapy with aspirin, beta-blockers, and statins. As a result, revascularization should only be considered for patients if they meet an indication for revascularization irrespective of their planned surgery.39
The revascularization options for patients with stable angina include both CABG and PCI. The landmark BARI (Bypass Angioplasty Revascularization Investigation) trial randomized 1,829 patients with multivessel coronary artery disease (patients with left main disease were excluded) to either PTCA (prior to the use of stents) or CABG. There was no difference in survival between the two groups at 5 years; however, post hoc analysis showed that patients with diabetes had a 15% absolute reduction in mortality when undergoing CABG.40 Based on the results of BARI, surgical therapy has historically been the preferred method of revascularization in patients with diabetes or three-vessel coronary artery disease.
The SYNTAX (Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial was designed to provide contemporary data to guide the type of revascularization for patients with severe coronary artery disease.41 This contemporary trial differed from the BARI trial by including patients with left main disease and/or three-vessel coronary artery disease and randomizing them to either CABG or complete revascularization with drug-eluting stents. In addition, the SYNTAX trial utilized a team approach that included both an interventional cardiologist and a cardiac surgeon to determine whether patients were a candidate for both percutaneous revascularization and CABG. This team calculated a SYNTAX score which takes into account calcification, tortuosity, and length for every stenosis >50% found in vessels more than 1.5 mm in diameter. The SYNTAX score, if used by trained personnel, can be a reproducible score that allows for direct comparison of the severity of coronary artery disease between patients (SYNTAX score <22 = low risk, 23 to 32 = intermediate risk, ≥33 = high risk). In the 4,337 patients randomized, there was no difference in all-cause mortality, stroke, or MI at 1 year between the two groups (PCI 7.7% vs. CABG 7.6%, p = 0.99); however, the primary endpoint that added repeat revascularization to the three other endpoints occurred more frequently in the patients treated with PCI (17.8% vs. 12.4%, p = 0.002). Post hoc analysis at 4 years showed that patients treated with CABG had lower cardiovascular mortality when compared to patients treated with PCI (4.3% vs. 7.6%, p = 0.004) although the validity of this finding is uncertain given the difference in the numbers of patients lost to follow-up seen between the two arms (CABG arm had more patients lost to follow-up). These results largely coincided with smaller randomized clinical trials that evaluated PCI with bare metal stents to CABG which suggested that there is no mortality benefit provided by PCI over CABG [ARTS-I (Arterial Revascularization Therapies Study Part I), MASS-II (Medicine, Angioplasty, or Surgery Study for Multivessel Coronary Artery Disease), ERACI-II (Argentine Randomized Study of Coronary Angioplasty with Stenting versus Coronary Bypass Surgery in Patients with Multiple Vessel Disease), and AWESOME (Angina with Extremely Serious Operative Mortality Evaluation)]. Patients with more severe coronary artery disease are likely to see lower rates of repeat revascularization after CABG; however, those patients with isolated left main disease and/or low SYNTAX score have improved outcomes with percutaneous revascularization.
In summary, CABG has previously been the preferred method of revascularization for patients with three-vessel coronary artery disease and LV dysfunction, left main disease, or diabetes and multivessel disease. With the potential exception of patients with diabetes, there has been no definitive mortality difference between the two types of revascularization. Patients undergoing CABG are at increased risk of perioperative stroke, while patients undergoing PCI are at increased risk of restenosis and the need for repeat revascularization. Drug-eluting stents have been successful in decreasing the rates of restenosis allowing for the percutaneous revascularization of the left main artery and patients with three-vessel coronary artery disease; however, the use of drug-eluting stents remains associated with higher rates of repeat revascularization. CABG provides the most enduring revascularization for patients with the most extensive coronary artery disease (SYNTAX > 22) (Table 47.8).
TABLE
47.8 ACC/AHA Appropriateness Criteria for the Selection Of PCI or CABG in Patients with Severe Coronary Artery Disease
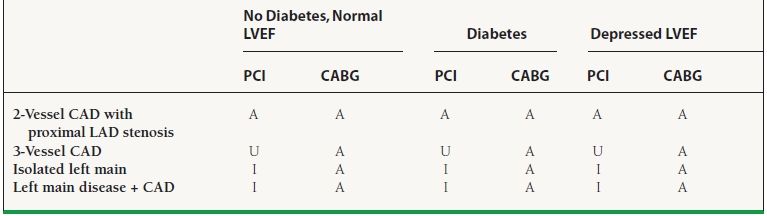
A, Appropriate; U, Appropriateness uncertain; I, Inappropriate.
Modified from Patel MR, Dehmer GJ, Hirshfeld JW, et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2009;53:530–553..
Patients Who Are Undergoing PCI Post-CABG
Patients who undergo CABG are still at risk of ischemia due to either graft occlusion or the progression of disease in the native vessels. Considering that repeat CABG carries a higher risk (approximately two times the risk of mortality in an otherwise low-risk patient), a post-CABG patient with recurrent ischemia may require PCI procedures to the grafts or the native vessels. The timing of ischemia following CABG offers insight into the etiology of the ischemia and the appropriateness for PCI in the management of this population. Ischemia within 30 days (very early ischemia) is usually secondary to graft failure from thrombosis or native vessel disease that was not supplied by the coronary bypass grafts. Ischemia occurring 30 days to 1 year following CABG is usually secondary to perianastomotic graft stenosis (early ischemia). Ischemia occurring >1 year after CABG (late ischemia) is usually secondary to disease progression in the grafts and/or native coronary vessels. PCI may be used to recanalize thrombosed vessels, dilate anastomotic points, or balloon or stent native vessels or grafts. It is important to keep in mind that the choice of PCI versus redo CABG in patients with recurrent ischemia depends on multiple factors including the potential graft conduits (arterial vs. venous), the number of grafts required and how many are occluded, the location of recurrent disease (in native vessels vs. grafts), LV function, and associated comorbidities.
ADJUNCT THERAPY FOR PCI
Antiplatelet Therapies
Aspirin/Thienopyridines
Early efforts at coronary stenting were hindered by high rates of stent thrombosis following PCI. Aspirin and thienopyridine have become the standard antiplatelet regimen used after PCI to prevent stent thrombosis after the conclusion of the STARS trial (Stent Anticoagulation Restonosis Study).42 The STARS trial randomized 1,653 patients with recent stent implantation to aspirin only, aspirin plus ticlopidine, or aspirin plus warfarin. At 30 days, only 0.5% of patients treated with aspirin and ticlopidine had the primary endpoint (death, MI, revascularization of the target lesion, and angiographically evident stent thrombosis) that was significantly less than both aspirin alone (3.6%) and aspirin plus warfarin (2.6%). The use of ticlopidine in clinical care was hindered by its side effect profile that included both neutropenia and thrombotic thrombocytopenic purpura. As a result, clopidogrel, which has a lower incidence of these side effects, has become the most used thienopyridine.43 The efficacy of clopidogrel in patients treated with PCI has been tested and shown to be effective in multiple studies. The CREDO (Clopidogrel for the Reduction of Events during Observation) trial randomized 2,116 patients undergoing PCI with bare metal stenting to either clopidogrel load with continued therapy for 1 year or clopidogrel after PCI with continued therapy for 28 days. Those patients treated with long-term clopidogrel had a 27% relative risk reduction in death, MI, or stroke at 1 year.44
Prasugrel is a new thienopyridine that has a faster onset and more potent antiplatelet effects than clopidogrel.45 It was approved for use in patients with acute coronary syndrome based on the results of the TRITON TIMI-38 trial.46 TRITON randomized 13,608 patients with acute coronary syndrome undergoing PCI to standard therapy with clopidogrel (300-mg loading dose and long-term treatment with 75 mg/d) or prasugrel (60-mg loading dose and long-term treatment with 10 mg/d). After a follow-up that ranged between 6 and 15 months, cardiovascular mortality, nonfatal MI, and nonfatal stroke were significantly lower in the group treated with prasugrel (hazard ratio for prasugrel vs. clopidogrel, 0.81; p < 0.001). There were some significant safety concerns that have limited the widespread adoption of this agent. Patients treated with prasugrel had a significant increase in bleeding (hazard ratio, 1.32; 95% CI, 1.03 to 1.68; p = 0.03) and patients with prior transient ischemic attack/cerebral vascular accident (TIA/CVA) had worse outcomes when treated with prasugrel. Also, patients older than 75 years and <60 kg did not appear to benefit from increased platelet inhibition. Based on this subgroup analysis, prasugrel is contraindicated in patients with prior TIA/CVA, age >75 years old, or <60 kg due to the increased bleeding risk.
Ticagrelor is a newly approved antiplatelet agent that is a direct and reversible inhibitor of the P2Y12 receptor. The half-life of the drug is 12 hours and as a result is given twice daily. The largest study of ticagrelor is the Study of Platelet Inhibition and Patient Outcomes (PLATO).47 The PLATO study randomized 18,624 patients with ACS to either ticagrelor (180-mg loading dose and then 90 mg twice daily) or clopidogrel (300- to 600-mg loading dose and then 75 mg daily). After 12 months, death from vascular causes, MI, or stroke was less common in the patients treated with ticagrelor (HR 0.84, p < 0.001).48 While the overall outcomes favored ticagrelor, there are some safety concerns. Among patients not undergoing CABG, bleeding rates were higher in patients treated with ticagrelor (4.5% vs. 3.8%, p = 0.03). In addition, a post hoc analysis demonstrated that patients randomized from the United States had worse outcomes with ticagrelor.49 It is unclear whether this is due to statistical chance or that patients in the United States were more likely to be on higher doses of aspirin. In summary, ticagrelor is an agent with antiplatelet effects that are reversible and have a short half-life. Given the potential interaction between high-dose aspirin and outcomes, ticagrelor should only be used in conjunction with low doses of aspirin.
The dosing and duration of dual antiplatelet therapy (DAPT) have changed over time. Current guidelines recommend that patients not already taking daily chronic aspirin therapy should be given 300 to 325 mg of aspirin before the PCI with long-term therapy at a dose between 75 mg and 325 mg/d. Further insight into the most appropriated aspirin dose has been provided with the CURRENT OASIS-7 trial. This trial randomized patients with ACS treated with an early invasive strategy in a two-by-two factorial design to low- versus high-dose aspirin and low- versus high-dose clopidgrel.50 At 30 days, there was no difference in the primary endpoint of cardiovascular death, MI, or stroke. Given the higher bleeding rates seen with higher doses of aspirin, it is reasonable to treat patients with the lowest available aspirin dose.51 Current ACC/AHA guidelines do not yet reflect the results of this trial. Despite concurrent thienopyridine, aspirin remains necessary for patients treated with PCI. Among patients with aspirin sensitivity, desensitization can be considered; however, thienopyridines alone are more frequently utilized.
The duration of clopidogrel treatment after PCI is also subject to some debate. Originally, it was recommended that patients treated with bare metal stent be treated for at least 4 weeks, while patients treated with drug-eluting stents be treated for 3 to 6 months depending on the type of stent. Over time, it became apparent that there was a small but significant incidence of late stent thrombosis in patients treated with drug-eluting stents.52 This was attributed to the lack of endothelialization caused by the medications on the stent designed to inhibit restenosis. Current guidelines support the use of DAPT for at least 12 months; however, there will be no definitive answer regarding the optimal duration of DAPT therapy until the completion of the ongoing DAPT Study (Dual Antiplatelet Therapy Study).53 This trial is enrolling patients treated with drug-eluting stents who will then be treated for 12 months with DAPT. After 12 months, patients will be randomized to either placebo (thereby completing DAPT) or an additional 18 months of DAPT (long- term DAPT). Scheduled for completion in December 2013, this trial will provide evidence that will be helpful in determining whether long-term DAPT past 1 year is necessary (Table 47.9).
TABLE
47.9 Recommendations for Adjunctive Antiplatelet Therapies in PCI

Adapted from the ACC/AHA guidelines: King SB III, Smith SC Jr, Hirshfeld JW Jr, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51:172–209; Smith SC Jr, Feldman TE, Hirshfeld JW Jr, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). Circulation. 2006;113:156–175; Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241.



