46 Percutaneous Closure of Patent Foramen Ovale and Atrial Septal Defect
 Introduction
Introduction
The advent of cardiopulmonary bypass support revolutionized the management of many structural cardiac abnormalities. Ever since the first surgical repair of an ASD in 1952, surgical techniques were refined steadily, which conferred excellent short-term as well as long-term outcomes. Until recently, the management of ASD was primarily surgical. The last two decades witnessed the growth of percutaneous techniques for the management of coronary and other vascular pathologies. The refinements in percutaneous interventional technology and recent advances in the cardiac imaging techniques permitted percutaneous treatment of selected structural cardiac defects. ASD, one of the most common congenital cardiac anomalies, was one of the earliest to be approached percutaneously. King and Mills first reported ASD closure in five patients in 1976 using a double umbrella device.1 They have since reported a 27-year follow-up on these five patients.2 This device required a very large delivery system, which limited its use. These early successes were followed by the Rashkind device and subsequently the Lock Clamshell Occluder in the late 1980’s.3,4 Improvements in the technology paved the way for a newer generation of devices that made percutaneous closure of a secundum ASD not only acceptable but also preferable to the surgical approach. The closely related entity patent foramen ovale (PFO), which, until now, was rarely treated surgically despite its known association with paradoxical embolism, also became accessible to percutaneous closure. The last 9 years since the U.S. Food and Drug Administration (FDA) approval of selected devices for closure of ASDs and PFOs have seen a paradigm shift in the management of these two entities. ASD and PFO shifted away from the surgical arena and into the catheterization laboratory (cath lab); because of this, patient recovery time was shortened, complications were decreased, and treatment efficacy was maintained. The association of PFO with other disease processes such as migraine was identified, and this opened the door to new treatment options for a large portion of patients. This chapter provides an introduction to the percutaneous closure of ASDs and PFOs. It includes an overview of their embryology, pathophysiology, and clinical associations, followed by a description of the available devices for closure. The procedure, its indications, and complications are also detailed.
 Embryology
Embryology
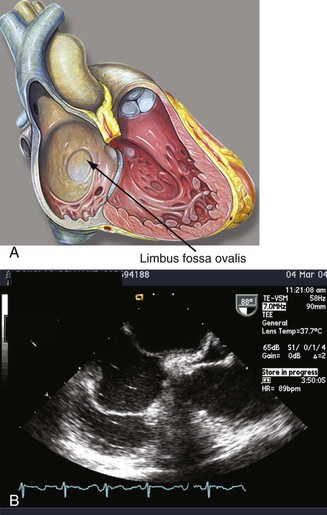
By the eighteenth day of gestation, the primordium of the heart becomes evident. At the end of the fourth week, the endocardial cushions fuse to form the right and left atrioventricular (AV) canals. The endocardial cushions will serve as the primordium of the AV valves and the inferior wall of the atrium. At this time, the common atrium undergoes a complicated process of septation. The septum primum grows in a caudal direction toward the endocardial cushions, thus closing the inter-atrial communication (ostium primum). As the septum primum reaches its destination, the cells in its superior portion undergo apoptosis and coalesce to form the ostium secundum. A muscular septum secundum forms to the right of the septum primum and extends to reach the caudal border of the ostium secundum, forming a “flap-like valve” between both atria (Fig. 46-1; see Video 46-1 ![]() ).5 Oxygenated placental blood enters the right atrium from the inferior vena cava (IVC) and is directed toward the IAS by the eustachian valve. The low left atrial pressure, the lack of blood flow through the pulmonary veins, and the preferential flow of the IVC to the IAS allows oxygenated blood to cross the foramen ovale and enter the systemic circulation. Blood entering the right atrium from the SVC is directed away from the IAS by the crista interveniens, preventing the mixture of nonoxygenated blood in this chamber. The right horn of the sinus venosus incorporates the SVC and the IVC into the right atrium (see Video 46-2
).5 Oxygenated placental blood enters the right atrium from the inferior vena cava (IVC) and is directed toward the IAS by the eustachian valve. The low left atrial pressure, the lack of blood flow through the pulmonary veins, and the preferential flow of the IVC to the IAS allows oxygenated blood to cross the foramen ovale and enter the systemic circulation. Blood entering the right atrium from the SVC is directed away from the IAS by the crista interveniens, preventing the mixture of nonoxygenated blood in this chamber. The right horn of the sinus venosus incorporates the SVC and the IVC into the right atrium (see Video 46-2 ![]() ). At birth, the pulmonary vascular resistances and the right cardiac pressures fall, and the left atrial pressure increases, forcing the septum primum against the septum secundum and occluding the “valve-like” foramen ovale. Complete occlusion occurs in the majority of the population, but in approximately 25% of the population, the fusion is incomplete, giving rise to a PFO.6
). At birth, the pulmonary vascular resistances and the right cardiac pressures fall, and the left atrial pressure increases, forcing the septum primum against the septum secundum and occluding the “valve-like” foramen ovale. Complete occlusion occurs in the majority of the population, but in approximately 25% of the population, the fusion is incomplete, giving rise to a PFO.6
 Patent Foramen Ovale
Patent Foramen Ovale
By echocardiography, the incidence of a PFO in the adult population is estimated to be approximately 25%.6 Autopsy studies revealed the presence of a probe-patent PFO of 0.2 to 0.5 cm in 29%.7 The frequency of PFO decreases with age and increases in size with each decade of life.8 Spontaneous PFO closure may occur during adulthood, although recent data suggest that PFOs may recanalize over time.9 The incidence rate of PFO is equal in both genders and among all ethnic groups; however, the PFOs in whites and Hispanics are larger and are associated with a greater degree of shunting.10 The presence of a PFO was thought to be inconsequential until 1877 when Cohnheim postulated that a venous thrombosis may paradoxically traverse a foramen ovale and give rise to a systemic embolism.11 Since that time, PFOs have been associated with varying disease processes, including cryptogenic stroke and paradoxical embolization, platypnea orthodeoxia syndrome, hypoxemia with normal pulmonary pressures, DCS in divers and high-altitude pilots, and migraine headaches.12–17
Patent Foramen Ovale and Cryptogenic Stroke
Depending on the age group, the etiology of a cerebrovascular accident (CVA) may vary. Atrial fibrillation (AF) and small vessel disease contribute to the majority of the strokes in patients older than 50 years of age. In patients younger than 35 years, the most common causes include nonatherosclerotic arteriopathies, arterial dissection, and thromboembolism.9 However, in 35% to 40% of patients who suffer CVAs, the etiologies remain unknown even after a thorough evaluation, and the CVAs are classified as cryptogenic. Under this classification, different etiologies that remain unidentified by current diagnostic modalities may be included. The search for the probable etiologies of cryptogenic strokes has generated conflicting information. In a retrospective case-control study, PFOs were seen to be four times more prevalent among young adults who experienced a stroke without an identifiable cause compared with others with known causes.13 In a meta-analysis, PFOs were found to occur up to six times more frequently among patients younger than 55 years who suffered a cryptogenic stroke compared with those who had identifiable causes of CVAs.18 Others have noted a higher frequency of PFOs in patients with cryptogenic strokes irrespective of age (<55 years 48% vs. 4%; >55 years 38% vs. 8%, P < 0.001), although this observation is not generally accepted.14 Among patients with cryptogenic strokes, patients with PFOs were less likely to have traditional cardiovascular risk factors such as hypertension, hypercholesterolemia, and tobacco use, which suggests a different mechanism for CVAs in this population subset.19 The mechanism by which a PFO may participate in the generation of a cryptogenic stroke is unclear. In situ thrombosis, paradoxical embolization, and predisposition to atrial arrhythmias have been proposed as mechanisms for PFO-associated cryptogenic strokes.20 Paradoxical embolization—the passage of a venous thrombus into the systemic circulation through a PFO—has been the predominant theory (see Video 46-3 ![]() ; Fig. 46-2). Evidence that supports the role of PFOs in cryptogenic strokes includes case reports of the transit of thrombi across PFOs; cerebral distribution of cryptogenic CVAs, which suggests an embolic nature; and the increased frequency of deep venous thrombosis (DVT) in patients who had cryptogenic CVAs.21–23 The Paradoxical Embolism from Large Veins in Ischemic Stroke (PELVIS) trial noted an increased frequency of positive magnetic resonance venography (MRV) testing for pelvic thrombus in patients with PFOs and cryptogenic CVAs compared with patients with known causes (20% vs. 4%, P < 0.03).24 The corollary that patients with pulmonary emboli have a significantly higher stroke rate in the presence of PFOs (13% vs. 2%, P = 0.02) is also true.25 In a prospective trial of 503 patients, Handke et al have shown that PFOs were more frequent in patients experiencing cryptogenic strokes irrespective of age (in patients younger than 55 years 43.9% vs. 14.3%; odds ratio [OR] 4.70, P < 0.001; and in patients older than 55 years 28.3% vs. 11.9%; OR 2.92, P < 0.001).26 These data underline the causative role of paradoxical embolization in patients who had cryptogenic strokes. However, this association is still being debated. The majority of the information available on the association of PFOs and cryptogenic strokes originates from small case-control or retrospective studies, which places constraints on drawing a definitive conclusion. A large population-based case-control study that included 1072 participants (random controls, patients who had suffered noncryptogenic strokes, and patients who had suffered cryptogenic strokes) failed to show an association between cryptogenic strokes and PFOs.27 The lack of association may have been related to the study design, since case selection included patients who did not have recurrent CVAs. Data from the SPARC trial, a prospective trial that questioned the veracity of a causal relationship between PFOs and strokes of unknown etiology, have been published.28 The trial included 588 healthy volunteers in Olmstead County, who underwent multi-modality testing and follow-up for stroke risk assessment. Over a period of 5 years, 41 of the subjects experienced a stroke. After adjusting for age and other cardiovascular comorbidities, PFOs were not found to be an independent predictor of stroke (hazard ratio [HR] 1.28; 95% confidence interval [CI] 0.65–2.50). The Kaplan Meier estimate of survival free of CVA was 91% and 93% in patients with and without PFOs, respectively. The trial confirms that the presence of a PFO does not pose an increased risk of stroke in asymptomatic patients. Unfortunately, this trial included an older population (66.9 ± 13 years) even though most other trials recognize the association between cryptogenic stroke and age in patients younger than 55 years; and this trial had a low prevalence of CVAs, which limited its ability to detect a statistically significant hazard. The hazard ratio may be as low as 0.65 or as high as 2.5 and still be consistent with the study’s findings.
; Fig. 46-2). Evidence that supports the role of PFOs in cryptogenic strokes includes case reports of the transit of thrombi across PFOs; cerebral distribution of cryptogenic CVAs, which suggests an embolic nature; and the increased frequency of deep venous thrombosis (DVT) in patients who had cryptogenic CVAs.21–23 The Paradoxical Embolism from Large Veins in Ischemic Stroke (PELVIS) trial noted an increased frequency of positive magnetic resonance venography (MRV) testing for pelvic thrombus in patients with PFOs and cryptogenic CVAs compared with patients with known causes (20% vs. 4%, P < 0.03).24 The corollary that patients with pulmonary emboli have a significantly higher stroke rate in the presence of PFOs (13% vs. 2%, P = 0.02) is also true.25 In a prospective trial of 503 patients, Handke et al have shown that PFOs were more frequent in patients experiencing cryptogenic strokes irrespective of age (in patients younger than 55 years 43.9% vs. 14.3%; odds ratio [OR] 4.70, P < 0.001; and in patients older than 55 years 28.3% vs. 11.9%; OR 2.92, P < 0.001).26 These data underline the causative role of paradoxical embolization in patients who had cryptogenic strokes. However, this association is still being debated. The majority of the information available on the association of PFOs and cryptogenic strokes originates from small case-control or retrospective studies, which places constraints on drawing a definitive conclusion. A large population-based case-control study that included 1072 participants (random controls, patients who had suffered noncryptogenic strokes, and patients who had suffered cryptogenic strokes) failed to show an association between cryptogenic strokes and PFOs.27 The lack of association may have been related to the study design, since case selection included patients who did not have recurrent CVAs. Data from the SPARC trial, a prospective trial that questioned the veracity of a causal relationship between PFOs and strokes of unknown etiology, have been published.28 The trial included 588 healthy volunteers in Olmstead County, who underwent multi-modality testing and follow-up for stroke risk assessment. Over a period of 5 years, 41 of the subjects experienced a stroke. After adjusting for age and other cardiovascular comorbidities, PFOs were not found to be an independent predictor of stroke (hazard ratio [HR] 1.28; 95% confidence interval [CI] 0.65–2.50). The Kaplan Meier estimate of survival free of CVA was 91% and 93% in patients with and without PFOs, respectively. The trial confirms that the presence of a PFO does not pose an increased risk of stroke in asymptomatic patients. Unfortunately, this trial included an older population (66.9 ± 13 years) even though most other trials recognize the association between cryptogenic stroke and age in patients younger than 55 years; and this trial had a low prevalence of CVAs, which limited its ability to detect a statistically significant hazard. The hazard ratio may be as low as 0.65 or as high as 2.5 and still be consistent with the study’s findings.
Stroke Recurrence and Risk Identification
Prospective data from an observational study presented in abstract form have documented the incidence of CVAs in patients with PFOs as 1.10 per 100 person-years and in patients without PFOs as 0.97 per 100 person-years.29 The low incidence does not make primary prevention cost effective. Additional risk factors that may detect people at risk need to be identified. The reported recurrence rate of cryptogenic stroke varies from 1.2% to greater than 16% but is generally around 2%.30,31 In retrospective studies, the risk of recurrence has been found to be related to PFO size, patency at rest, shunt severity, and the presence of atrial septal aneurysm (ASA).32–34 A prominent eustachian valve has been associated with increased patency of the foramen ovale and the risk of stroke recurrence, as it preferentially directs blood flow to the IAS (see Video 46-4 ![]() ; Fig. 46-3).35 It has been postulated that a mobile IAS may increase the size of the foramen ovale facilitating the passage of thrombi. Recurrences of cryptogenic CVAs have been found to be associated with the degree of septal protrusion: patients with septum excursion greater than 6.5 mm had a risk of recurrence of 12.3% versus 4.3% at 3 years.30 The combination of ASA and PFO was associated with an increased risk of recurrence in the French PFO/ASA study (HR 4.17; 95% CI 1.47–11.84).36 However, this finding was not supported by the PICSS (PFO in Cryptogenic Stroke Study) trial.37 The PICSS trial was designed to define the rate of recurrent stroke or death in patients with and without PFOs, who were randomly treated with aspirin or warfarin over a period of 2 years. The multi-center, double-blind study included 265 patients who had cryptogenic strokes and 365 who had noncryptogenic strokes. PFOs were more frequently found in the cryptogenic stroke group (48% vs. 38%, P < 0.02), and larger PFOs were more frequently associated with the same stroke subtype (20% vs. 9.7%, P < 0.001). There was no statistically significant difference in the time to recurrent stroke or death in the cryptogenic stroke group when categorized by PFO status (14.3% vs. 12%). In all these patients, there was no difference in stroke recurrence when categorized by the presence of PFOs and ASAs. However, this study has significant limitations that may alter the generalization of its results. The subjects were older, had higher prevalence of CVA risk factors (diabetes and hypertension), and only 42% of the enrolled patients had had cryptogenic strokes. Because of this, the subgroup analysis of patients who had cryptogenic stroke may be underpowered for treatment assumptions. Because of the inherent limitations of the study design, the small patient population, and the differences in control groups, the association between PFOs and CVAs cannot be established at the present time. Larger randomized trials are required to answer this question.
; Fig. 46-3).35 It has been postulated that a mobile IAS may increase the size of the foramen ovale facilitating the passage of thrombi. Recurrences of cryptogenic CVAs have been found to be associated with the degree of septal protrusion: patients with septum excursion greater than 6.5 mm had a risk of recurrence of 12.3% versus 4.3% at 3 years.30 The combination of ASA and PFO was associated with an increased risk of recurrence in the French PFO/ASA study (HR 4.17; 95% CI 1.47–11.84).36 However, this finding was not supported by the PICSS (PFO in Cryptogenic Stroke Study) trial.37 The PICSS trial was designed to define the rate of recurrent stroke or death in patients with and without PFOs, who were randomly treated with aspirin or warfarin over a period of 2 years. The multi-center, double-blind study included 265 patients who had cryptogenic strokes and 365 who had noncryptogenic strokes. PFOs were more frequently found in the cryptogenic stroke group (48% vs. 38%, P < 0.02), and larger PFOs were more frequently associated with the same stroke subtype (20% vs. 9.7%, P < 0.001). There was no statistically significant difference in the time to recurrent stroke or death in the cryptogenic stroke group when categorized by PFO status (14.3% vs. 12%). In all these patients, there was no difference in stroke recurrence when categorized by the presence of PFOs and ASAs. However, this study has significant limitations that may alter the generalization of its results. The subjects were older, had higher prevalence of CVA risk factors (diabetes and hypertension), and only 42% of the enrolled patients had had cryptogenic strokes. Because of this, the subgroup analysis of patients who had cryptogenic stroke may be underpowered for treatment assumptions. Because of the inherent limitations of the study design, the small patient population, and the differences in control groups, the association between PFOs and CVAs cannot be established at the present time. Larger randomized trials are required to answer this question.
 Diagnosis of a Paradoxical Embolic Stroke
Diagnosis of a Paradoxical Embolic Stroke
The diagnosis of a cryptogenic stroke is a diagnosis of exclusion. A complete evaluation to rule out other causes must be performed before assigning this diagnosis and should include a hypercoagulable evaluation, echocardiogram, heart rhythm evaluation, and carotid Doppler, as detailed in Table 46-1. If the working diagnosis of paradoxical embolization is still feasible, then a right-to-left shunt should be evaluated with an echocardiogram with bubble study or a trans-cranial Doppler.
TABLE 46-1 Evaluation of Cryptogenic Stroke
| Condition | Diagnostic Test |
|---|---|
| Cerebrovascular disease | Carotid Doppler Magnetic resonance angiography |
| Cardiac source of embolism | |
| Left atrial appendage thrombus Left ventricular aneurysm | Trans-esophageal echocardiogram Trans-thoracic or trans-esophageal echocardiogram |
| Ascending aorta atheroma | Trans-esophageal echocardiogram |
| Paroxysmal atrial fibrillation | Holter monitor |
| Hypercoagulable state | Protein C and S activity Anti-thrombin III level Lupus anticoagulant Anticardiolipin antibody Factor V Leiden Prothrombin 20210A mutation |
Treatment for Cryptogenic Stroke
Medical Treatment
Controversy exists regarding the best method for the prevention of recurrent events in patients who have experienced cryptogenic strokes. Medical treatment with aspirin or oral anticoagulants has been reported. In the PFO–ASA study, 267 patients who experienced cryptogenic strokes and had PFOs only or PFOs with ASA were treated with aspirin or with aspirin and warfarin if they had a venous thrombosis.36 After 4 years of follow-up, there were 12 episodes of recurrent strokes and 9 recurrent transient ischemic attacks (TIAs). All episodes occurred in patients treated with aspirin. In the Lausanne Study, the 140 patients with the same characteristics were followed up for 36 months. Treatment was assigned on the basis of number of risk factors and included surgical PFO closure (8%), oral anticoagulation with target international normalized ratio (INR) of 3 to 4 (26%), or aspirin (66%). There was 1.9% yearly event rate for CVAs and 3.8% for the combination of CVAs and TIAs.38 A meta-analysis, which included 895 patients in the medical therapy arm, evaluated the benefit of medical therapy versus trans-catheter closure of PFOs in patients with presumed paradoxical embolization; the analysis yielded a 1-year recurrence rate between 3.8% and 12%.39 The PICSS trial was the only prospective, blinded, randomized trial that compared the efficacy of aspirin versus oral anticoagulation in patients with CVAs.38 It found no statistically significant difference in stroke recurrence between patients with PFOs treated with aspirin and those treated with warfarin (17.9% vs. 9.5%; HR 0.52; 95% CI 0.16–1.67, P = 0.28). The high recurrence rate suggests other (non-PFO) mechanisms for CVAs in the older population. As expected, there was a slight increase in the rate of minor hemorrhage in patients on the warfarin arm. Currently, there is no consensus on the superiority of anti-platelet or oral anticoagulation therapy in patients with cryptogenic strokes and PFOs.40
Surgical Treatment
Surgical closure of PFOs in patients who had cryptogenic strokes has been reported. The largest series included 91 consecutive patients with a mean age of 44 years and one prior CVA. Surgery was evaluated with intraoperative trans-esophageal echocardiography (TEE), and suture or patch closure was employed. Closure was achieved in 98% of cases, and the actuarial freedom from recurrence was 93% at 1 year and 83% at 4 years. The surgical procedure was associated with significant morbidity in 21%.41 Other smaller series reflected similar closure rates and significant morbidity.
Percutaneous Treatment
Percutaneous closure of PFOs has been available for the last decade. There have been no completed randomized trials to evaluate the superiority of percutaneous closure over medical treatment. The available data reflect single-operator or multiple-center experiences with moderate follow-up. Procedural success has been greater than 90% and may depend on the closure device used and associated anatomic variants. The presence of an ASA may decrease the rate of successful closure. Device modifications as well as growing experience and familiarity with the technique have greatly reduced the complication rate while maintaining appropriate closure rates. Windeker et al reported on 80 patients with a procedural success of 98% and a 2.5% recurrent TIA rate at 5 years.42 Residual shunting has been identified as a risk for recurrent CVAs; overall percutaneous closure has a recurrent rate similar to that of medical therapy.43,44 The only series that compared medical therapy to percutaneous closure in patients with PFOs and cryptogenic strokes was published by Windeker.45 Patients were nonrandomly assigned to percutaneous closure (n = 150) or medical treatment with aspirin (n = 78) or oral anticoagulation (n = 78) with an INR of 2 to 3. They were followed for 2.3 ± 1.7 years. Groups were comparable in terms of age, gender, and cardiovascular risk factors. Percutaneous closure led to a lower risk of the combined endpoint of death, recurrent stroke, or TIA (8.5% vs. 24%; RR = 0.48; 95% CI 0.23–1.01, P = 0.05) Patients with more than one event at baseline and those with complete occlusion of the foramen ovale were at lower risk of recurrent stroke or TIA after percutaneous treatment compared with those receiving medical therapy (7.3% vs. 33.2%; 95% CI 0.008–0.81, P = 0.01; and 6.5% vs. 22%4; 95% CI 0.14–0.99, P = 0.0). With its inherent limitations, this study demonstrated an advantage of percutaneous treatment in high-risk patients (recurrent CVAs), and hence the trials evaluating PFO closure have been developed as superiority trials and not as noninferior trials. Percutaneous closure of PFOs in patients who experienced recurrent CVAs despite being on medical therapy carries a class IIb indication by the American Heart Association (AHA) guidelines on stroke prevention.46 Data to support PFO closure after a first stroke are insufficient. The FDA has permitted the use of the Amplatzer and Cardioseal septal occluder device under humanitarian device exemption (HDE) only in patients who have experienced recurrent CVAs while on conventional medical therapy (oral anticoagulation with a therapeutic INR). Currently, two prospective trials that are aimed at comparing medical treatment with percutaneous closure of foramen ovale in patients with recurrent cryptogenic strokes are under way in the United States. Both CLOSURE-1 (Evaluation of the STARFlex Septal Closure system in patients with strokes or TIAs caused by the possible passage of clot of unknown origin through a patent foramen ovale), and RESPECT (Randomized evaluation of recurrent stroke comparing PFO closure with treatments with established current standard of care) are facing slow enrollment because of the widespread use of the HDE clause and placement of an ASD occluder or other devices in an off-label manner. It is strongly recommended that physicians refer their patients who may be eligible for enrollment in the trial so that the appropriate questions are answered. This HDE has been replaced with a registry for patients who do not meet the criteria for the RESPECT trial.
Migraine Headache
Migraine headaches affect 12% of the U.S. population and generate significant morbidity and economic burden. The etiology of migraine headache with aura has remained elusive. People with migraine headaches have a twofold increase in the risk of stroke, and the risk increases to 3.5 in patients younger than 35 years of age.47 Observational studies have also revealed that patients who experience migraines have increased frequency of silent, deep, white-matter lesions.48 Migraine headaches are more frequently seen in patients younger than 45 years of age who had experienced an infarct in the posterior cerebral circulation.49 These facts, and the growing information obtained from the evaluation of PFOs and cryptogenic strokes, prompted researchers to assess the frequency of right-to-left shunting in migraineurs. Anzola evaluated the presence of right-to-left shunting by trans-cranial Doppler (TCD) in 113 patients with migraine with aura and compared these patients with 53 patients with migraine without aura and 25 healthy age-matched controls. The presence of a PFO was significantly higher in the migraineurs with aura compared with the migraineurs without aura or the controls (48% vs. 23% vs. 20%).50 Retrospective analysis of patients who underwent PFO closure for cryptogenic strokes revealed a decrease in the frequency of attacks of migraine with aura in 80% or even complete resolution in 56%.51,52 Unfortunately, these studies may have been influenced by recall bias; the therapeutic effect of aspirin or clopidogrel, which may have been used for migraine prophylaxis; a high placebo effect; and the fact that migraine frequency decreases with age. In addition, retrospective studies do not demonstrate a causal association. The high frequencies of both PFOs and migraine headaches in the general population may favor a spurious association. Anzola et al prospectively compared patients with PFOs and cryptogenic strokes who underwent PFO closure (n = 23) with patients who had migraines, peripheral embolic events, or TIAs who underwent PFO closure (n = 27), and patients with migraines and PFOs who were treated medically (n = 27). After a 12-month follow-up, the frequency and intensity of the migraine attacks were significantly decreased in patients who underwent PFO closure.53 Nonrandomized registries of patients suffering from migraines with aura have shown a 70% to 90% improvement or symptom resolution after successful PFO closure.54–56 The association between migraine headache with aura and PFOs has generated new hypotheses on the etiology of migraines. It is now postulated that migraines may be related to microembolic events or the presence of high concentrations of circulating vasoactive substances that are not filtered by the lung, since they cross to the systemic circulation through PFOs. The MIST (Migraine Intervention With STARFlex Technology) trial attempted to test the association between PFOs and migraine headaches with aura. It was a multi-center, blinded study that randomized 432 patients with migraine to PFO closure with a STARFlex Septal Repair (NMT Medical, Boston, MA) implant or a sham procedure. The primary endpoint of cessation of migraines was not met. However, reduction in headache days in at least 50% occurred more frequently in the PFO closure group (42% vs. 23%, P = 0.038).57 The discrepancy between the results from the MIST trial and other registries may be related to the exclusion of patients with previous CVAs or TIAs and the lack of complete PFO closure in the MIST trial. The exclusion of patients with previous TIAs or CVAs potentially filtered out those most likely to experience paradoxical embolization and clinical benefit. There is an ongoing attempt to study such patients in a randomized fashion. The U.S.-based MIST II trial (NMT Medical, Boston, MA) and the ESCAPE (St. Jude Medical, Fullerton, CA) trial have been discontinued because of slow enrollment. The PREMIUM trial (AGA Medical, Golden Valley, MN) has been enrolling since 2006, will have longer follow-up, and will attempt to answer questions left unanswered on previous attempts. Until these results are available, PFO closure should not be considered for the treatment of migraine headache with aura. The Gore Helex PFO closure device may be evaluated in patients with migraine who have had CVAs or TIAs (Personal communication, Mark Reisman, MD, April 2010).
Platypnea–Orthodeoxia and Hypoxia
PFO has also been associated with the rare platypnea–orthodeoxia syndrome. Platypnea refers to the feeling of dyspnea when in an upright posture, and orthodeoxia is arterial desaturation that occurs on standing. It is postulated that right-to-left shunting occurs across a patent foramen, particularly if an ASA is present. This entity is seen primarily in the very old and is associated with an event that alters the geometry of intra-thoracic organs, such as a pneumonectomy or an enlarged ascending aorta. Extrinsic compression of the right atrium or decreased compliance of the right ventricle may also predispose to shunting at the atrial level in these patients.58 It is postulated that in patients with an elongated aorta, the heart is shifted laterally so that the IVC drains directly toward the atrial septum, although this is not fully understood as yet. This anatomic shift maintains the PFO open throughout the cardiac cycle and generates the physical findings.59 Diagnosis is made by using saline contrast echocardiography with the patient in the supine and seated positions.11 Surgical or percutaneous closure has been done successfully, leading to marked improvement in the patient’s symptoms.60,61 Hypoxia related to PFOs may also be observed in patients with severe pulmonary hypertension (PH) or obstructive sleep apnea.62 The mechanism involves transient or persistent elevation of the right atrial pressure in relation to the left atrial pressure or re-direction of the IVC blood flow toward the IAS. Hypoxia related to right-to-left shunting at the atrial level has been associated with pulmonary arteriovenous malformations (AVMs), liver disease, amiodarone toxicity, pulmonary emboli with transient pulmonary hypertension, positive pressure ventilation, hypovolemia, aortic aneurysm, right ventricular infarction, Ebstein’s anomaly, and carcinoid valve disease, among others. Making the diagnosis may be challenging, as it requires documentation of right-to-left shunting while the patient is hypoxic, and frank improvement occurs after closure. In patients with severe PH with decreased right ventricular function, the PFO serves as an escape valve, which aids the emptying of the right atrium into a lower pressure circuit (left atrium). In these patients, PFO closure may be fatal.
Decompression Sickness
DCS is caused by the presence of nitrogen bubbles that come out of solution in blood as the ambient pressure decreases when a person ascends from a dive. The amount of nitrogen bubbles generated will depend on the total time spent in the dive, the speed of ascent, compliance with decompression stops, and individual factors such as cardiac output. Generally, the nitrogen bubbles stay in the venous circulation and make it to the lungs, where they are rapidly diffused. It has been postulated that in the presence of a PFO, the nitrogen bubbles may enter the systemic circulation and travel superiorly toward the brain, occluding a small arterial branch. DCS in patients with PFOs is associated with early onset of cerebral or vestibular symptoms that occur within 30 minutes after a dive, even after the person has performed all the appropriate rest stops.63 The association of PFOs and DCS is relatively new. In a case control study, Germonpre noted an OR of 2.25 for the development of DCS in divers with PFOs.64 In a small case control study, patients with PFOs had an OR to develop DCS and were more likely to have silent ischemic lesions as shown on brain MRI.65 Torti noted that divers with PFOs had a higher risk of developing DCS, required treatment for DCS, and had DCS that lasted more than 24 hours.66 Currently, PFO closure to prevent DCS is not indicated because of low overall incidence and ease of avoidance of DCS.
Diagnosis
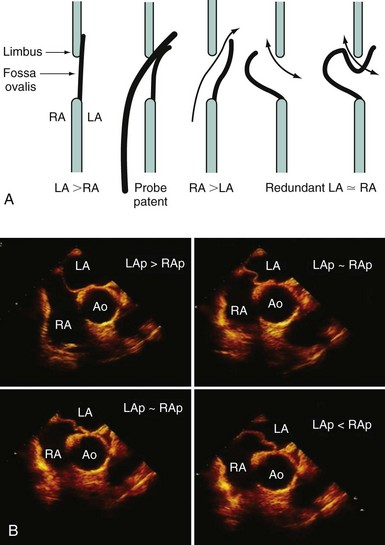
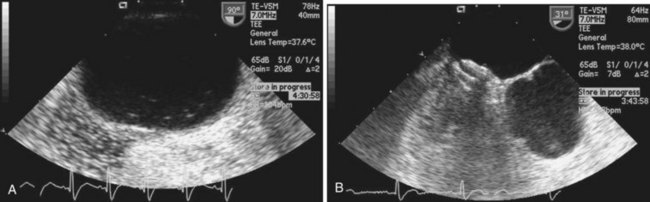
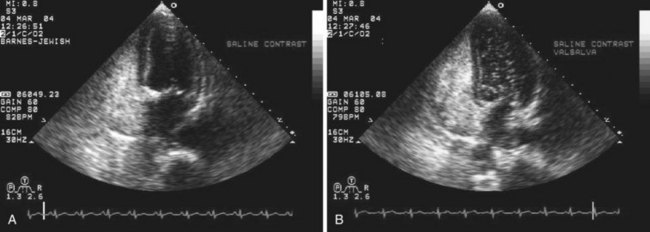
Echocardiography plays an important role in the diagnosis of abnormalities of the atrial septum. Traditionally, TEE has been considered the gold standard to diagnose a PFO. The advantage of TEE is that it can identify all portions of the IAS, allowing for the diagnosis of all subtypes of ASDs, a fenestrated atrial septum, and PFOs. TEE also allows for the detailed identification of lipomatous hypertrophy of the septum, atrial septal aneurysms, a prominent eustachian valve, or a long PFO tunnel that may alter a planned closing procedure (see Fig. 46-3; see Video 46-5 ![]() ). TEE can identify other potential sources of embolization (i.e., left atrial appendage thrombus, cardiac tumors, aortic atheroma, etc.) as well. An ASA is defined as a redundance of the atrial septum with excursion greater than10 mm into either of the atria and a 15-mm base. The degree and direction of the inter-atrial shunt will depend on the net pressure difference between both atria. The inter-atrial shunt direction will change with the phase of respiration and the cardiac cycle. It can be documented by color Doppler interrogation of the IAS (Fig. 46-4). Color interrogation along the fossa ovalis may cause erroneous identification of a PFO because of color cross-contamination when lowering the Nyquist limit. TEE’s diagnostic sensitivity is significantly lower than that of a saline-contrasted study; the addition of saline contrast improves TEE’s diagnostic sensitivity.67 The injection of saline contrast through the femoral vein has been shown to be superior for the diagnosis of PFOs by TEE and for the appropriate sizing of ASDs.68 Appropriate provocative measures that transiently increase the right atrial pressure (Valsalva maneuver), may be difficult to perform during a TEE because of the patient’s sedation, relative hypovolemia from a fasting state, and the inability to close the glottis against the echo probe (Fig. 46-5; see Videos 46-6 through 46-8
). TEE can identify other potential sources of embolization (i.e., left atrial appendage thrombus, cardiac tumors, aortic atheroma, etc.) as well. An ASA is defined as a redundance of the atrial septum with excursion greater than10 mm into either of the atria and a 15-mm base. The degree and direction of the inter-atrial shunt will depend on the net pressure difference between both atria. The inter-atrial shunt direction will change with the phase of respiration and the cardiac cycle. It can be documented by color Doppler interrogation of the IAS (Fig. 46-4). Color interrogation along the fossa ovalis may cause erroneous identification of a PFO because of color cross-contamination when lowering the Nyquist limit. TEE’s diagnostic sensitivity is significantly lower than that of a saline-contrasted study; the addition of saline contrast improves TEE’s diagnostic sensitivity.67 The injection of saline contrast through the femoral vein has been shown to be superior for the diagnosis of PFOs by TEE and for the appropriate sizing of ASDs.68 Appropriate provocative measures that transiently increase the right atrial pressure (Valsalva maneuver), may be difficult to perform during a TEE because of the patient’s sedation, relative hypovolemia from a fasting state, and the inability to close the glottis against the echo probe (Fig. 46-5; see Videos 46-6 through 46-8 ![]() ). Fundamental imaging trans-thoracic echocardiography (TTE) has been considered inferior for the diagnosis of PFOs. However, the advent of second harmonic imaging has improved the sensitivity of TTE to 90%.69 An easier and more effective performance of a provocative maneuver (no sedation, euvolemia, complete glottic closure) during TTE may improve the image quality and is associated with a higher sensitivity than that of TEE for the diagnosis of PFOs.70 In addition, the lack of invasiveness makes TTE a more attractive screening tool for PFOs (Fig. 46-6).
). Fundamental imaging trans-thoracic echocardiography (TTE) has been considered inferior for the diagnosis of PFOs. However, the advent of second harmonic imaging has improved the sensitivity of TTE to 90%.69 An easier and more effective performance of a provocative maneuver (no sedation, euvolemia, complete glottic closure) during TTE may improve the image quality and is associated with a higher sensitivity than that of TEE for the diagnosis of PFOs.70 In addition, the lack of invasiveness makes TTE a more attractive screening tool for PFOs (Fig. 46-6).
Trans-cranial Doppler (TCD) also has a role in the detection and quantification of right-to-left shunt. TCD insonates the middle cerebral artery and detects the presence of high-intensity transient signals when injected through a vein. Its sensitivity is similar to that of TEE, but its major limitation is its inability to detect the origin of the shunt. The number of transient signals has been shown to correlate with PFO size and postprocedure PFO patency.67,71
Devices
Amplatzer Patent Foramen Ovale Occluder
The Amplatzer PFO occluder is a self-expanding, double-disk device made from 0.005-inch nitinol wire, with a polyester fabric sewn into both disks (Fig. 46-7). There are three device sizes based on the right atrial disk diameter—18 mm, 25 mm, and 35 mm. In contrast to the ASD occluder, the stem is thin and mobile. The right atrial arm is larger than the left arm in the 25 mm and 35 mm sizes, whereas both are equal in the 18 mm. The right atrial disk stabilizes the device, thus preventing embolization from right to left. The thin stem allows varying degrees of disk mobility that permits the PFO occluder to seat appropriately in a long tunnel or around a hypertrophied septum. The left atrial disk dimensions are designed to decrease interference with pulmonary venous drainage or with the mitral valve and to minimize the presence of thrombogenic material in the systemic circulation. Device sizing will depend on the measurement from the foramen ovale to the SVC and from the foramen ovale to the aorta. The right disk radius should not exceed the shortest distance obtained (Table 46-2). The 25-mm device is used in the vast majority of the cases. The Amplatzer PFO occluder has the advantage of being self-expanding, has a simple deployment, and is placed through a small venous sheath (8F for 18 mm and 25 mm, and 9F for 35 mm). It is fully retractable until it is released. Its major disadvantage is that it is bulky within the atrial septum.
TABLE 46-2 Measurement for Device Selection for AGA Patent Foramen Ovale Occluder
| Distance from Defect to SVC or Aorta (mm) | Suggested Device | Delivery Sheath |
|---|---|---|
| >17.5 | 35 mm | 9F |
| 12.5–17.4 | 25 mm | 8F |
| 9–12.4 | 18 mm | 8F |
| <9 | None |
F, French; mm, millimeters; SVC, superior vena cava.
STARFlex Septal Occluder
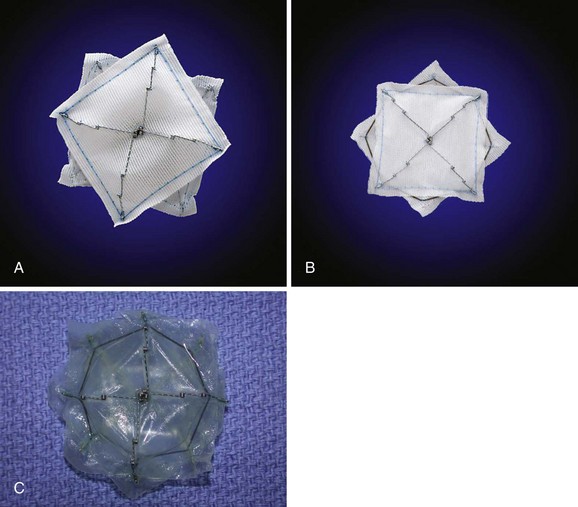
The STARFlex septal occluder is characterized by a self-centering mechanism made from a single nitinol microspring, which attaches each arm to the corresponding arm of the opposing umbrella (Fig. 46-8). Available sizes are 23 mm, 28 mm, and 33 mm and the use of each is determined by PFO balloon sizing. Its major advantage is its low profile. Although modifications in the delivery system have been made, device retrieval may be cumbersome. It was used in the CLOSURE-I trial, which has completed enrollment.
Premere Patent Foramen Ovale Closure System
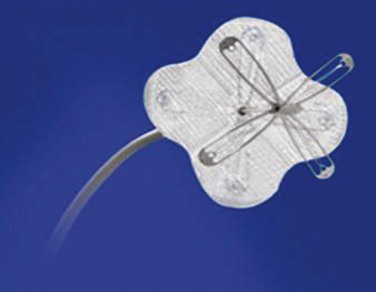
The Premere PFO closure system was available to patients participating in the PFO/Migraine trial sponsored by St Jude’s Medical (Fig. 46-9), which has now been discontinued. The Premere system has a very small profile and may be difficult to detect with echocardiography. Placement requires balloon sizing. Its unique design allows the operator to increase tension between the left and right atrial components by pulling the suture linking the two components together until locked, allowing it to conform to various tunnel lengths. Its major disadvantage is that larger venous sheaths (11F) and many steps are required for closure. The operator has to be extremely careful not to excessively tighten the knot that holds the two disks together, since excessively tightened knots cannot be loosened subsequently and may lead to device fracture or malapposition.
Future Devices
The next generation of devices will be aimed at reducing the material that persists within the atria after closure. Initial trials using a bioabsorbable device, BioSTAR by NMT Medical, have been completed, and the device is currently available in Europe (see Fig. 46-8). Alternative methods of closure are also being developed and include suture closure (Sutura Inc.) or tissue cauterization with radiofrequency (Coaptus RF (CoAptus Medical Inc.) and PFx (Cierra Inc.) (Fig. 46-10); these methods have been evaluated with inconsistent results. The Coherex device (Coherex Medical, Salt Lake, UT, USA) (Fig. 46-11), which uses technology to selectively close the PFO tunnel without leaving significant material in the atrial chambers has been approved for commercial use in Europe. The Gore Helex (Gore Medical, AZ, USA) (Fig. 46-12) device is approved for ASD closure and, along with the Cribriform (Amplatzer, MN USA) device, has been used off label for PFO closure (Figs. 46-13 and 46-14).

Figure 46-10 Photograph of the PFx closure catheter with radiofrequency ablation.
(Courtesy of Cierra, Inc.)