Percutaneous Bypass and Future Applications
Alan C. Yeung
Joshua Makower
Theodore C. Lamson
Since Andreas Gruntzig’s pioneering work gave birth to the field of interventional cardiology, great strides have been made to reduce the invasiveness of therapies for the treatment of coronary artery disease (CAD). Although over a million CAD patients are effectively treated percutaneously each year in the United States, an additional quarter to half million still remain who undergo highly invasive coronary bypass surgery, and again as many patients exist who are deemed “nonrevascularizable” and thus receive suboptimal therapy (1). In recent years, efforts have been pursued to develop novel procedures that effect percutaneous “nonsurgical” coronary and peripheral artery bypass (2, 3, 4, 5).
In this chapter, we discuss several of these novel procedures and the devices that make them possible. In the coronary vasculature, clinical experience in percutaneous coronary artery bypass has been limited to percutaneous in situ coronary venous arterialization (PICVA). This bypass technique relies on retroperfusion of the capillary network via anastomosis of a coronary artery with its adjacent coronary vein. Additionally, preclinical work has been pursued with VPASS, a variant of PICVA in which oxygenated blood is sourced directly from the ventricle via a ventricle-to-vein channel. The ultimate procedure to achieve artery-to-artery bypass in the coronary artery is percutaneous in situ coronary artery bypass (PICAB). This “true” bypass has been achieved in a porcine model.
In the peripheral vasculature, great strides also have been made recently in effecting a true artery-toartery bypass.
PERCUTANEOUS IN SITU CORONARY VENOUS ARTERIALIZATION (PICVA)
In 2001, we reported the first ever percutaneous bypass procedure, performed in Trier, Germany (2) (Fig. 22.1). With the advent of drug-eluting stents (DESs) and other important advancements in stent-related technologies that have improved the interventionalist’s ability to treat chronic total occlusions, the initial focus on percutaneous coronary bypass was directed toward the treatment of patients in the limited-option or no-option population (6). Due to the diffuse nature of their coronary disease, bypass surgery and even DESs are unlikely to provide adequate myocardial perfusion in these patients. Although various angiogenic and vasculogenic strategies are under development (e.g., gene and cell therapy), they face a fundamental challenge: Although such therapies may be capable of increasing the microvascular density within a tissue, this effect may have little impact on perfusion unless the inflow of oxygenated blood to the area of vasculogenesis is also increased.
In PICVA, the coronary venous segment that typically drains the area of ischemic myocardium is arterialized by being linked to brisk arterial inflow. Thus, ischemia may be relieved by oxygenated blood reaching the myocardium via retroperfusion of the venules and capillary bed. Considerable support exists for this concept in a series of clinical investigations spanning several decades of research (7, 8, 9, 10).
The PICVA procedure requires a series of novel catheters and implantable devices, originally designed and developed by TransVascular, Inc. and now being further advanced by Medtronic (Santa Rosa, California). These enabling devices can be divided into four categories:
Coronary sinus guiding catheters, developed to access the coronary sinus from a femoral approach. Because electrophysiologic access to the coronary sinus is primarily transjugular, it was necessary to develop custom shapes for access from the femoral approach. One form of coronary sinus guide catheter has an integrated occlusion balloon to facilitate retrograde injection of contrast media into the coronary venous system.
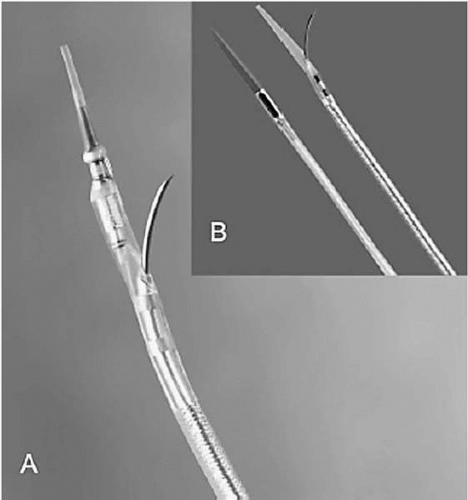
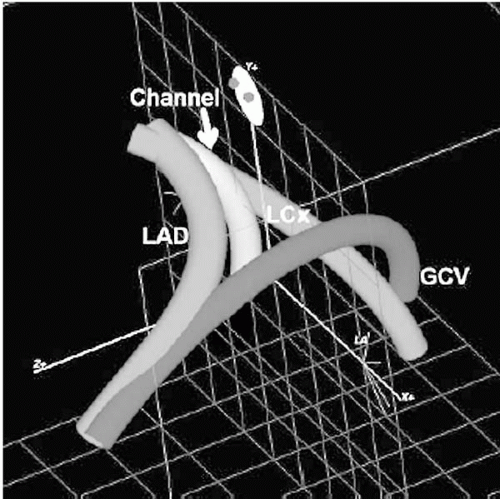
TransAccess catheter, which is essentially the platform technology for all the percutaneous bypass procedures currently under development. The TransAccess catheter is an image-guided needle delivery catheter, designed, in the case of percutaneous bypass, to accurately deliver a guidewire from one vascular structure to another. Numerous versions of this technology have been employed, but the majority of experience has been with catheters incorporating an integrated phased-array intravascular ultrasound (IVUS) guidance system (Fig. 22.2A), supplied by Volcano, Inc. (Rancho Cordova, California). The TransAccess catheter is guided into position under fluoroscopy, and the IVUS is used to accurately target the vascular structure for guidewire delivery. In a later series of PICVA procedures, three-dimensional navigational systems were used for vessel targeting. In place of the IVUS scanner, a Biosense Webster NOGA sensor assembly (Biosense Webster, a Johnson & Johnson Company, Diamond Ban, California) was incorporated into the tip of the TransAccess catheter, and an over-the-wire NOGA-based Target catheter was developed (Fig. 22.2B). Again, fluoroscopy was used to guide the TransAccess and Target catheters into position, then the NOGA system was used to position the TransAccess catheter so that the needle would be deployed toward the Target catheter (Fig. 22.3).
Blocking devices and delivery systems, for occlusion of the natural venous, egress toward the coronary sinus, thus redirecting oxygenated blood retrograde in the vein toward the area of myocardial ischemia (retroperfusion). The devices used in the original clinical series were self-expanding covered stents, delivered much like an embolization coil (Fig. 22.4A). In a later clinical series, a balloon-expandable system, the Vetour (TransVascular, Inc.), was employed. This device consisted of a stainless steel stent structure partially covered by expanded polytetrafluoroethylene (ePTFE), with a cell size chosen to promote thrombotic occlusion. After stent deployment, as the balloon is retracted, a self-contracting
nitinol tip cinches down the ePTFE covering to effect total occlusion of the vessel (Fig. 22.4B).
Anastomotic connectors, designed for delivery over the TransAccess-placed 0.014-inch guidewire and used to secure a permanent connection between the vessels. The majority of PICVA cases were completed with the delivery of a self-expanding system (TransVascular, Inc.) designed specifically for coronary artery-vein anastomosis (Fig. 22.5), but in one case, a Jostent covered stent (Abbott Laboratories, Redwood City, California) was employed. Although the delivery aspects of the Jostent are challenging, the covered nature of the device obviates the need for a blocker.
In addition to these novel devices, standard angioplasty guides, guidewires, and percutaneous transluminal coronary angioplasty (PTCA) balloon catheters are used in the PICVA procedure.
The PICVA procedure begins with a standard coronary artery injection using late-phase imaging to observe the delayed venous filling, opacifying the coronary sinus and coronary venous tree. A 14 Fr coronary sinus guide catheter is inserted into the femoral vein in tandem with a 7 Fr SIM-shaped diagnostic catheter over a 0.035-inch guidewire, and the assembly is advanced to the right atrium. The diagnostic catheter is manipulated into the coronary sinus, the guidewire then is advanced into the great cardiac vein (GCV), and the 14 Fr guide then is advanced over the diagnostic catheter into position in the coronary sinus.
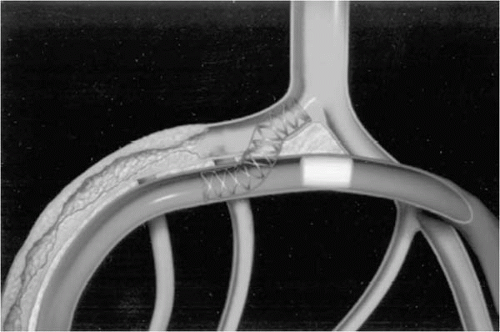
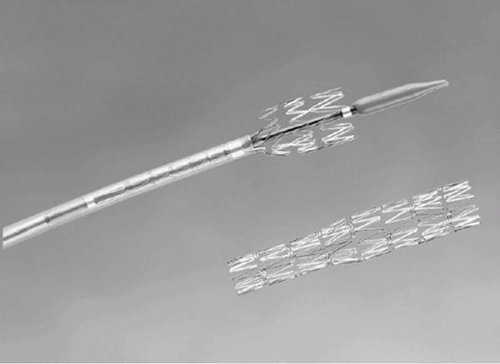 Figure 22.5. Self-expanding connector, implanted to preserve the fistulous tract created between coronary artery and vein. Constrained on its 5 Fr delivery catheter (left), the connector is implanted by retracting the exterior sheath. The larger-caliber portion of the implant (right) accommodates the larger luminal diameter of the native cardiac vein, whereas the smaller caliber portion conforms to the arterial lumen diameter. (Reproduced with permission from Oesterle SN, Reifart N, Hayase M, et al. Catheter-based coronary bypass: a development update. Catheter Cardiovasc Interv 2003;58(2):212-218.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|