Chapter 13 Percutaneous Biopsy Procedures
Techniques and Indications
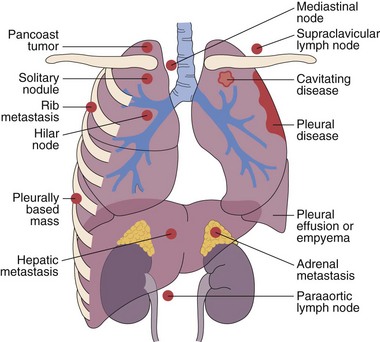
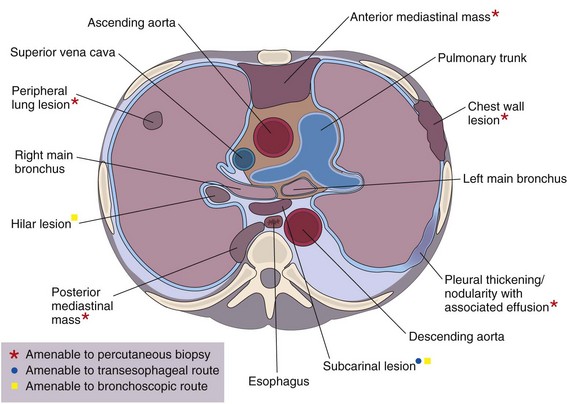
This chapter describes the techniques and equipment available to obtain diagnostic samples from intrathoracic and selected extrathoracic lesions by the percutaneous route. The common lesions sampled and their locations are listed in Figure 13-1. The most frequent methods of sampling intrathoracic lesions, depending on their site, are summarized in Figure 13-2. The techniques and indications are different from those required to diagnose more diffuse intrapulmonary disease. The latter, which includes conditions such as idiopathic pulmonary fibrosis, requires a transbronchial lung biopsy or an open lung biopsy through a mini-thoracotomy or video-assisted thoracoscopy (VATS) procedure.
Methods of Tissue Sampling
Imaging Techniques
Integrated Positron Emission Tomography–Computed Tomography
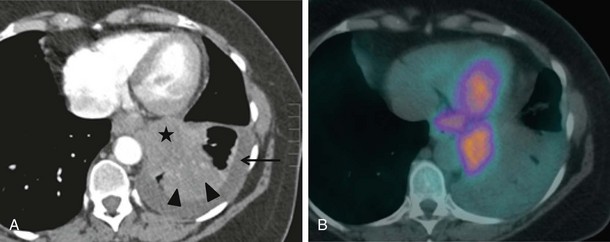
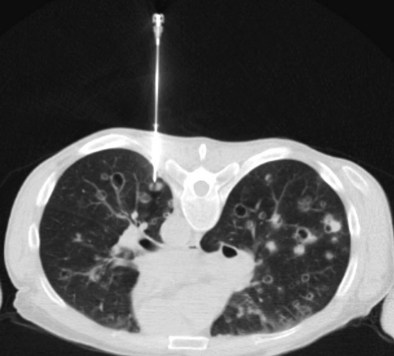
CT combined with PET allows accurate anatomic localization of FDG-avid foci. This technique is particularly useful in isolating active foci surrounded by benign changes, such as within thickened pleura, or in separating tumor from collapse, allowing greater precision in positioning the biopsy needle (Figure 13-3).
Needles
The two main modes of sampling are fine needle aspiration (FNA) for cytologic study and core biopsy for histopathologic examination. Both methods retrieve samples that are suitable for culture. Although the sensitivity of FNA is improved by having a cytologist present to ensure that an adequate sample is obtained, the diagnostic yield in benign disease remains low (20% to 50%) compared with that for core biopsy (70%) (Greif et al., 1999). In malignant disease, the techniques are analogous, with a sensitivity of 90% to 95% (Klein et al., 1996). The clinical requirement for cores of tissue for immunohistochemical analysis has led to a reduction in the use of FNA, and when feasible, cores are always obtained.
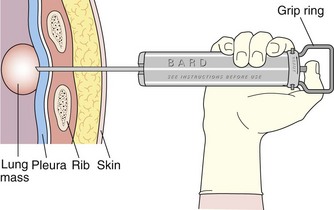
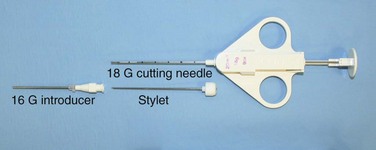
Core biopsy specimens are obtained using cutting needles, which are larger in diameter (14 to 18 gauge) and are available in different lengths (6-, 9-, and 15-cm lengths are commonly used). These needles are more sturdy, allowing greater control in placement. Cutting needles are typically mounted on a spring-loaded mechanism. Older designs such as the Bard Biopty biopsy system, when triggered, simultaneously fire an inner notched stylet and an outer cutting cannula. The handle of the Bard system is bulky but reusable (Figure 13-4). Newer, lighter designs include the Cook Quickcore and Bauer Temno devices, which allow the inner notched stylet to be advanced and secured within the lesion before the cutting cannula is activated. The throw can be increased from 1 to 2 cm to obtain better core samples (Figure 13-5). The Cook device seems to have a slightly sharper needle tip, which in practice can be advanced through tougher tissues.
Historically, it was believed that use of larger cutting needles carried higher complication rates than those associated with fine needles. A 2002 study of practice based in the United Kingdom analyzed data from 5444 lung biopsy and FNA procedures and found no difference between the two methods, a conclusion supported by other studies (Richardson et al., 2002).
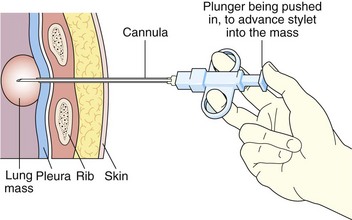
With transpulmonary biopsy, the risk of pneumothorax is more closely related to the number of pleural passes. The introduction of a coaxial system has transformed lung biopsies. A single pleural pass is made with a thin-walled introducer needle (usually 16 gauge). A smaller cutting needle (usually 18 gauge with a 1- or 2-cm throw) is inserted through the introducer and multiple cores are taken without repuncturing the pleura. The process is quicker, simpler, and safer than attempting multiple pleural passes, leading to a reduction in the complication rate with an improved diagnostic yield (Figure 13-6).
Percutaneous Biopsy of Intrapulmonary Lesions
Indications
The role of percutaneous lung biopsy in diagnosing malignant disease is well established, with a sensitivity of 90% to 95%. Its main application is in patients with inoperable lung cancer, when sputum cytology and bronchoscopy are nondiagnostic, to provide a means of establishing cell type before chemotherapy and radiotherapy. In the past, both FNA and core biopsy have been comparable in diagnosing and distinguishing small cell lung cancer (SCLC) from non-SCLC (NSCLC), providing oncologists with sufficient information to make choices regarding appropriate chemotherapy regimens. New targeted chemotherapy and biologic agents (e.g., the epidermal growth factor receptor [EGFR] inhibitor drugs) have proven prognostic benefit with particular histologic subtypes. This has meant that core sampling is essential to allow routine performance of detailed tissue analysis. FNA can be adequate to differentiate between subtypes of NCSLC, but it remains less accurate overall than core biopsy. The latter allows sufficient material to be obtained for both accurate histologic subtyping and molecular testing (e.g., to determine EGFR mutation status) (Barnes et al., 2010).
Biopsy also is widely used to exclude lung metastases in patients with potentially operable lung tumors, and in those with extrathoracic malignancies (Figure 13-7).
Contraindications
The contraindications to percutaneous lung biopsy are largely relative and are summarized in Table 13-1. In general, patients need to be able to cooperate, including lying still in the desired position, resisting excessive coughing, and controlling breathing. In patients with very poor lung function, biopsy of a lesion is still possible, provided that the lesion is peripheral and a carefully considered route is identified (usually with CT) that does not traverse lung parenchyma. In performing the biopsy, care is taken to avoid creating a pneumothorax, which could be life-threatening. Before the procedure, a coagulation screen is performed according to local protocol, and bleeding diatheses are corrected, when appropriate, to keep within safe guidelines. Biopsy of parenchymal hydatid lesions has been documented, but an increased and probably unacceptable risk of anaphylactic reaction has been documented in patients with such lesions.
Table 13-1 Contraindications to Needle Biopsy
| Type of Contraindication | Comment |
|---|---|
| Relative | Inability of patient to cooperate: uncontrollable cough, inability to lie prone or supine |
| Poor lung function/chronic obstructive pulmonary disease (FEV1 <40% of predicted normal or multiple bullae) | |
| Pneumonectomy | |
| Bleeding disorder | |
| Pulmonary hypertension | |
| Pulmonary fibrosis | |
| Small nodules (<5 mm in diameter) | |
| Hydatid disease (associated with risk of anaphylactic reaction) | |
| Absolute | Arteriovenous malformation with high pulmonary artery pressure |
FEV1, forced expiratory volume in 1 second.
Procedure
Practical Aspects
Radiopaque surface skin markers are placed 0.5 to 1.0 cm apart over the region of interest, and a limited CT scan covering the area is performed with the patient in gentle respiration (Figure 13-8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree