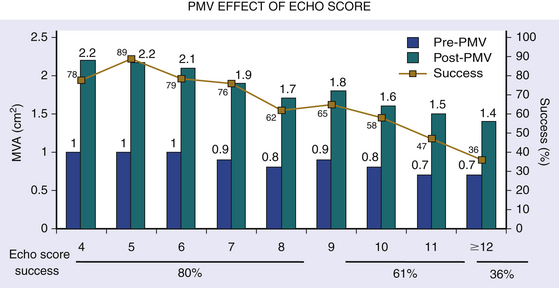
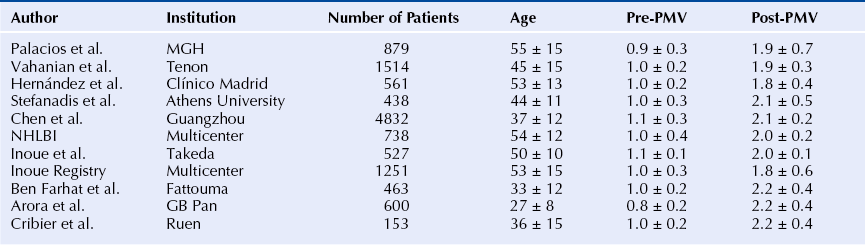
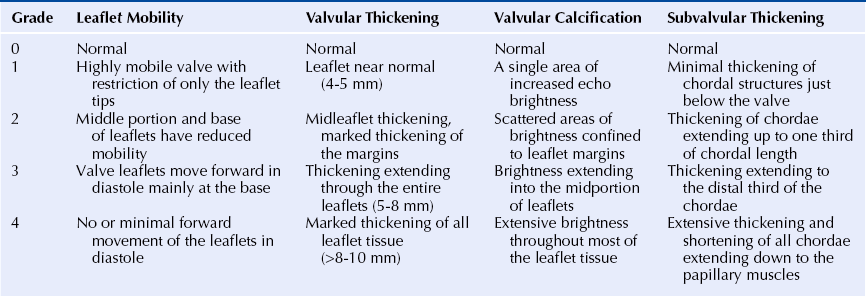
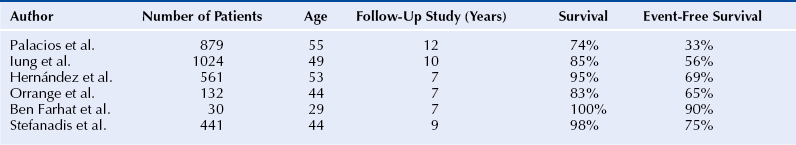
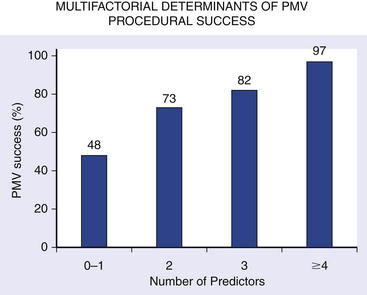
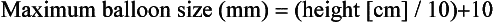Chapter 9 Before 1982, cardiac surgery was the conventional form of treatment for symptomatic stenotic valvular heart disease lesions.1,2 Today, percutaneous balloon dilation of stenotic cardiac valves is being used in many centers for the treatment of patients with pulmonic, mitral, aortic, and tricuspid stenosis. Since its introduction in 1984 by Inoue et al.,3 percutaneous mitral balloon commissurotomy or percutaneous mitral valvuloplasty (PMV) has been used successfully as an alternative to open or closed surgical mitral commissurotomy for the treatment of patients with symptomatic rheumatic mitral stenosis.4–18 PMV produces good immediate hemodynamic outcome, low in-hospital complication rates, and clinical improvement in the majority of patients with rheumatic mitral stenosis. PMV is safe and effective, and it provides sustained clinical and hemodynamic improvement in patients with rheumatic mitral stenosis. The immediate and long-term results appear to be similar to those of surgical mitral commisssurotomy.19–26 PMV is the preferred form of therapy for relief of mitral stenosis for a selected group of patients with symptomatic rheumatic mitral stenosis. Selection of patients for PMV should be based on symptoms, physical examination, and two-dimensional (2D) and Doppler echocardiographic findings.4,17,27,28 PMV is usually performed electively. However, emergency PMV can be performed as a life-saving procedure in patients with mitral stenosis and severe pulmonary edema refractory to medical therapy and/or cardiogenic shock. Patients considered for PMV should be symptomatic (New York Heart Association [NYHA] ≥ II), should have no recent thromboembolic events, have less than two grades of mitral regurgitation (MR) by contrast ventriculography using the Sellers’ classification,29 and have no evidence of left atrial thrombus on 2D and transesophageal echocardiography (Table 9–1). Transthoracic and transesophageal echocardiography should be performed routinely before PMV. Patients in atrial fibrillation and patients with previous embolic episodes should have treatment with warfarin to achieve anticoagulation with a therapeutic international normalized ratio (INR) for at least 3 months before PMV. Patients with left-atrium thrombus on 2D-echocardiography should be excluded. However, PMV could be performed in these patients if left-atrium thrombus has resolved after warfarin therapy. TABLE 9–1 Recommendations for Percutaneous Mitral Valvuloplasty∗ NYHA, New York Heart Association. ∗Adapted from currently American College of Cardiology/American Heart Association and European Guidelines for the management of patients with valvular heart disease. A multifactorial score derived from clinical, anatomic/echocardiographic, and hemodynamic variables can predict procedural success and clinical outcome (Figure 9–1).28 Demographic data, echocardiographic parameters (including echocardiographic score, Figure 9–2), and procedure-related variables recorded from 1085 consecutive patients who underwent PMV at the Massachusetts General Hospital (MGH) and their long-term clinical follow-up data (i.e., death, mitral valve replacement, redo PMV) were used to derive this clinical score. Multivariate regression analysis of the first 800 procedures was performed to identify independent predictors of procedural success. Significant variables were formulated into a risk score and validated prospectively. Six independent predictors of PMV success were identified: (1) age younger than 55 years, (2) NYHA classes I and II, (3) pre-PMV mitral area of 1 cm2 or greater, (4) pre-PMV MR grade less than 2, (5) echocardiographic score of 8 or less, and (6) male gender.4,27,28 A score was constructed from the arithmetic sum of variables present per patient. Procedural success rates increased incrementally with increasing score (0% for a score of 0/6, 39.7% for 1/6, 54.4% for 2/6, 77.3% for 3/6, 85.7% for 4/6, 95% for 5/6, and 100% for 6/6; p < 0.001). In a validation cohort (n = 285 consecutive procedures), the multifactorial score remained a significant predictor of PMV success (p < 0.001). Comparison between the new score and the echocardiographic score confirmed that the new index is more sensitive and specific (p < 0.001). This new score also predicts long-term outcomes (p < 0.001). Clinical, anatomic, and hemodynamic variables predict PMV success and clinical outcome and may be formulated in a scoring system that would help to identify the best candidates for PMV. Figure 9–1 A multifactorial score derived from clinical, anatomic/echocardiographic, and hemodynamic variables would predict procedural success and clinical outcome. PMV, Percutaneous mitral balloon commissurotomy. Hemodynamic measurements, cardiac output, and cine left ventriculography are performed before and after PMV. Cardiac output is measured by thermodilution and Fick method techniques. Mitral valve calcification and angiographic severity of MR (Sellers’ classification) are graded qualitatively from 0 to 4 as previously described.29 An oxygen diagnostic run is performed before and after PMV to determine the presence of left-to-right shunt across the atrial septum after PMV. There is not a unique technique of percutaneous mitral balloon valvuloplasty. Most of the techniques of PMV require transseptal left heart catheterization and use of the antegrade approach.15–24,26,30–32 Antegrade PMV can be accomplished using double balloon technique (Figure 9–3, A), or the more commonly used single balloon technique (Figure 9–3, B). In this latter approach the two balloons could be placed through a single femoral vein and single transseptal puncture or through two femoral veins and two separate atrial septal puncture. In the retrograde technique of PMV the balloon dilating catheters are advanced percutaneously through the right and left femoral arteries over guidewires that have been snared from the descending aorta. These guidewires have been advanced transseptally from the right femoral vein into the left atrium, the left ventricle, and the ascending aorta.31 A retrograde nontransseptal technique of PMV has also been described.15 A technique of PMV using a newly designed metallic valvulotome has also been introduced.16 The device consists of a detachable metallic cylinder with two articulated bars screwed onto the distal end of a disposable catheter, on which the proximal end is connected to activating pliers. Squeezing the pliers opens the bars up to a maximum of 40 mm (see Figure 9–3, C). The results with this device are at least comparable to those of the other balloon techniques of PMV.16 However, multiple uses after sterilization should markedly decrease procedural costs. Figure 9–3 Different percutaneous approaches of percutaneous mitral balloon commissurotomy. In performing PMV using the antegrade double balloon technique (see Figure 9–3, A) two 0.0038-inch (260-cm) Teflon-coated exchange wires are placed across the mitral valve into the left ventricle, through the aortic valve into the ascending and then the descending aorta (see Double Balloon Antegrade PMV Technique).4,18–19 Care should be taken to maintain large and smooth loops of the guidewires in the left ventricular cavity to allow appropriate placement of the dilating balloons. If a second guidewire cannot be placed into the ascending and descending aorta, a 0.038-inch Amplatz guidewire (Cook Inc., Bloomington, Ind.) with a preformed curl at its tip can be placed at the left ventricular apex. In patients with an aortic valve prosthesis, both guidewires with preformed curl tips should be placed at the left ventricular apex. When one or both guidewires are placed in the left ventricular apex, the balloons should be inflated sequentially. Care should be taken to avoid forward movement of the balloons and guidewires to prevent left ventricular perforation. Two balloon-dilating catheters are chosen according to the patient’s body surface area such that the ratio of effective balloon-dilating area (EBDA) to body surface area is greater than 3.1 cm2/m2 and less than 4 cm2/m2 (Table 9–2). The balloon valvotomy catheters are then advanced over each one of the guidewires and positioned across the mitral valve parallel to the longitudinal axis of the left ventricle. The balloon valvotomy catheters are then inflated by hand until the indentation produced by the stenotic mitral valve is no longer seen. Generally one, but occasionally two or three, inflations are performed. After complete deflation, the balloons are removed sequentially. PMV can also be performed using the Inoue technique (see Figure 9–3, B).3 The Inoue balloon is a 12F-shaft, coaxial, double-lumen catheter. The balloon is made of a double layer of rubber latex tubing with a layer of synthetic micromesh in between. After transseptal catheterization, a stainless steel guidewire is advanced through the transseptal catheter and placed with its tip coiled into the left atrium, and the transseptal catheter is removed. A 14F dilator is advanced over the guidewire and used to dilate the femoral vein and the atrial septum. A balloon catheter is chosen according to the patient’s height using the formula: The balloon catheter is advanced over the guidewire into the left atrium. The distal part of the balloon is partially inflated and advanced into the left ventricle with the help of the spring wire stylet, which has been inserted through the inner lumen of the catheter. The stylet is rotated in a counterclockwise fashion with gentle backward traction on the catheter. Once the catheter is in the left ventricle, the distal aspect of the balloon is inflated and moved back and forth inside the left ventricle to assure that it is free of the chordae tendineae. The catheter is then gently pulled against the mitral plane until resistance is felt. The balloon is then rapidly inflated to its full capacity and deflated quickly (Videos 9–1 and 9–2, Video 9–1, Video 9–2). The mechanism of successful PMV is splitting of the fused commissures toward the mitral annulus, resulting in commissural widening. This mechanism has been demonstrated by pathological, surgical, and echocardiographic studies.27,33 In addition, in patients with calcific mitral stenosis the balloons could increase mitral valve flexibility by the fracture of the calcified deposits in the mitral valve leaflets.33 Although rare, undesirable complications such as leaflet tears, left ventricular perforation, tear of the atrial septum, and rupture of chordae, mitral annulus, and papillary muscle could also occur. Figure 9–3 shows the hemodynamic changes produced by PMV in one patient. PMV resulted in a significant decrease in mitral gradient, mean left atrium pressure, and mean pulmonary artery pressure, and an increase in cardiac output and mitral valve area (MVA). Table 9–3 shows the changes in MVA reported by several investigators using different techniques of PMV. In most series, PMV is reported to increase MVA from less than 1.0 cm2 to approximately 2.0 cm2.4,12–18,22,34 Eight hundred and seventy nine consecutive patients with mitral stenosis underwent 939 consecutive PMVs at MGH between July 1986 and July 2000.4 As shown in Figure 9–4, in this group of patients PMV resulted in a significant decrease in mitral gradient from 14 ± 6 to 6 ± 3 mmHg. The mean cardiac output significantly increased from 3.9 ± 1.1 to 4.5 ± 1.3 L/min, and the calculated MVA increased from 0.9 ± 0.3 to 1.9 ± 0.7 cm2. In addition, mean pulmonary artery pressure significantly decreased from 36 ± 13 to 29 ± 11 mmHg, and the mean left atrial pressure decreased from 25 ± 7 to 17 ± 7 mmHg; consequently, the calculated pulmonary vascular resistances decreased significantly after PMV. Figure 9–4 Hemodynamic changes produced by a successful PMV in one patient with severe mitral stenosis. Simultaneous left atrium and left ventricular pressures before (left panel) and after (right panel) PMV. The corresponding calculated MVAs are also displayed. LA, Left atrium; LV, left ventricle; MVA, mitral valve area; PMV, percutaneous mitral balloon valvuloplasty. A successful hemodynamic outcome (defined as a post-PMV MVA ≥1.5 cm2 and post-PMV MR <3 Sellers grade) was obtained in 72% of the patients. Although a suboptimal result occurred in 28% of the patients, a post-PMV MVA of 1.0 cm2 or less (critical MVA) was present in only 8.7% of these patients. Univariate analysis demonstrated that the increase in MVA with PMV is directly related to the balloon size employed because it reflects in the EBDA, and inversely related to the echocardiographic score (see Figure 9–2), the presence of atrial fibrillation, the presence of fluoroscopic calcium, the presence of previous surgical commissurotomy, older age, NYHA classification pre-PMV, and presence of MR before PMV. Multiple stepwise regression analysis identified balloon size (p < 0.02), the echocardiographic score (p < 0.0001), and the presence of atrial fibrillation (p < 0.009) and MR before PMV (p < 0.03) as independent predictors of the increase in MVA with PMV.4 Univariate predictors of procedural success included age, pre-PMV MVA, mean pre-PMV pulmonary artery pressure, male gender, echocardiographic score, pre-PMV MR of 2+ or greater, history of previous surgical commissurotomy, presence of atrial fibrillation, and presence of mitral valve calcification under fluoroscopy.4 Multiple stepwise logistic regression analysis identified larger pre-PMV MVA (odds ratio [OR] 13.05; 95% confidence interval [CI] 7.74 to 22.51; p < 0.001), less degree of pre-PMV MR (OR 3.85; CI 2.27 to 6.66; p < 0.001), younger age (OR 3.33; CI 1.41 to 7.69; p = 0.006), absence of previous surgical commissurotomy (OR 1.85; CI 1.20 to 2.86; p = 0.004), male gender (OR 1.92; CI 1.19 to 3.13; p = 0.008), and echocardiographic score of 8 or lower (OR 1.69; CI 1.18 to 2.44; p = 0.004).4 The echocardiographic examination of the mitral valve can accurately characterize the severity and extent of the pathologic process in patients with rheumatic mitral stenosis. The most utilized score to identify the anatomic abnormalities of the stenotic mitral valve is that described by Wilkins et al. (see Figure 9–2 and Table 9–4).27,35,36 This echocardiographic score is an important predictor of the immediate and long-term outcome of PMV. In this morphologic score, leaflet rigidity, leaflet thickening, valvular calcification, and subvalvular disease are scored from 0 to 4. A higher score represents a heavily calcified, thickened, and immobile valve with extensive thickening and calcification of the subvalvular apparatus. The increase in MVA with PMV is inversely related to the echocardiographic score. The best outcomes with PMV occur in those patients with echocardiographic scores of 8 or lower. The increase in MVA is significantly greater in patients with echocardiographic scores of 8 or higher than in those with echocardiographic scores higher than 8 (Videos 9–3 and 9–4, Video 9–3, Video 9–4). The increase in MVA with PMV is directly related to balloon size. This effect was first demonstrated in a subgroup of patients who underwent repeat PMV. They initially underwent PMV with a single balloon, resulting in a mean MVA of 1.2 ± 0.2 cm2. They underwent repeat PMV using the double balloon technique, which increased the EBDA normalized by body surface area from 3.41 ± 0.2 to 4.51 ± 0.2 cm2/m2. The mean MVA in this group after repeat PMV was 1.8 ± 0.7 cm2. The increase in MVA in patients who underwent PMV at MGH using the double balloon technique (EBDA of 6.4 ± 0.03 cm2) was significantly greater than the increase in MVA achieved in patients who underwent PMV using the single balloon technique (EBDA of 4.3 ± 0.02 cm2).38 The mean MVAs were 1.9 ± 0.7 and 1.4 ± 0.1 cm2 for patients who underwent PMV with the double balloon and the single balloon techniques, respectively. However, as previously mentioned, care should be taken in the selection of dilating balloon catheters so as to obtain an adequate final MVA and no change or a minimal increase in MR. The immediate outcome of patients undergoing PMV is inversely related to the severity of valvular calcification seen by fluoroscopy. Patients without fluoroscopic calcium have a greater increase in MVA after PMV than patients with calcified valves. Patients with either no or 1+ fluoroscopic calcium have a greater increase in MVA after PMV (1.1 ± 0.6 and 0.9 ± 0.5 cm2, respectively) than those patients with 2+, 3+, or 4+ fluoroscopic calcium (0.8 ± 0.6, 0.8 ± 0.5, and 0.6 ± 0.4 cm2, respectively).39 Although the increase in MVA with PMV is inversely related to the presence of previous surgical mitral commissurotomy, PMV can produce a good outcome in this group of patients. The post-PMV mean MVA in 154 patients with previous surgical commissurotomy was 1.8 ± 0.7 cm2, compared with a valve area of 1.9 ± 0.6 cm2 in patients without previous surgical commissurotomy (p < 0.05). In this group of patients an echocardiographic score of 8 or lower was an important predictor of a successful hemodynamic immediate outcome.40 The immediate outcome of PMV is directly related to the age of the patient. The percentage of patients obtaining a good result with this technique decreases as age increases. A successful hemodynamic outcome from PMV was obtained in fewer than 50% of patients 65 years or older.30,41 This inverse relationship between age and the immediate outcome from PMV is the result of the higher frequency of atrial fibrillation, calcified valves, and higher echocardiographic scores in elderly patients. The increase in MVA with PMV is inversely related to the presence of atrial fibrillation; the post-PMV MVA of patients in normal sinus rhythm was 2.0 ± 0.7 cm2, compared with a valve area of 1.7 ± 0.6 cm2 of those patients in atrial fibrillation. The inferior immediate outcome of PMV in patients with mitral stenosis who are in atrial fibrillation is more likely related to the presence of clinical and morphological characteristics such as advanced age, echocardiographic scores greater than 8, NYHA functional class IV, calcified mitral valves under fluoroscopy, and a previous history of surgical mitral commissurotomy.42 The presence and severity of MR before PMV is an independent predictor of unfavorable outcome of PMV. The increase in MVA after PMV is inversely related to the severity of MR determined by angiography before the procedure. This inverse relationship between the presence of MR and immediate outcome of PMV is in part caused by the higher frequency of atrial fibrillation, higher echocardiographic scores, calcified mitral valves under fluoroscopy, and older age in patients with MR before PMV.28,42 Table 9–5 shows the complications reported by several investigators after PMV.4,12–18,22,34 Mortality and morbidity with PMV are low and similar to surgical commissurotomy. Overall, there is a less than 1% procedural mortality. Severe MR (4 grades by angiography) has been reported in 1% to 5.2% of patients, with some of these patients requiring in-hospital mitral valve replacement. Thromboembolic episodes and stroke have been reported in 0% to 3.1% and pericardial tamponade in 0.2% to 4.6% of cases in these series. Pericardial tamponade can occur from transseptal catheterization and more rarely from ventricular perforation. PMV is associated with a 3% to 16% incidence of left-to-right shunt documented by oxymetry immediately after the procedure. However, the pulmonary-to-systemic flow ratio is greater than or equal to 2:1 in only a minimum number of patients. TABLE 9–5 Complications after Percutaneous Mitral Valvuloplasty MR, Mitral regurgitation; NHLBI, National Heart, Lung, and Blood Institute. Severe MR (4 grades by angiography) occurs in about 3% of patients undergoing PMV.28,42 An undesirable increase in MR (≥2 grades by angiography) occurred in 10.1% of patients. This undesirable increase in MR is well tolerated in most patients. Furthermore, more than half of them have less MR at follow-up cardiac catheterization. The ratio of the EBDA to body surface area is a predictor of increased MR after PMV.28,42,43 The EBDA is calculated using standard geometric formulas (see Table 9–2). The incidence of MR is lower if balloon sizes are chosen so that EBDA/body surface area is less than or equal to 4.0 cm2/m2. The single balloon technique results in a lower incidence of MR but provides less relief of mitral stenosis than the double balloon technique. Thus there is an optimal EBDA between 3.1 and 4.0 cm2/m2 that achieves a maximal MVA with a minimal increase in MR. An echocardiographic score for the mitral valve that can predict the development of severe MR after PMV has also been described.35 This score takes into account the distribution (even or uneven) of leaflet thickening and calcification, the degree and symmetry of commissural disease, and the severity of subvalvular disease (Table 9–6). TABLE 9–6 The Echocardiographic Score∗ for Severe Mitral Regurgitation after Percutaneous Mitral Commissurotomy ∗The total score is the sum of each of these echocardiographic features (maximum 16). Left-to-right shunt through the created atrial communication determined by oximetry occurred in 3% to 16% of patients undergoing PMV. The size of the defect is small as reflected in the pulmonary-to-systemic flow ratio of less than 2:1 in the majority of patients. Older age, fluoroscopic evidence of mitral valve calcification, higher echocardiographic score, pre-PMV lower cardiac output, and higher pre-PMV NYHA functional class are the factors that predispose patients to develop left-to-right shunt post-PMV.44 Clinical, echocardiographic, surgical, and hemodynamic follow-up study of patients with post-PMV left-to-right shunt demonstrated that the defect closed in approximately 60% of patients. Persistent left-to-right shunt at follow-up study is small ( Long-term follow-up studies after PMV are encouraging.4,22–26,30,46–60 After PMV, the majority of patients have marked clinical improvement and are scored at NYHA Class I or II. The symptomatic, echocardiographic, and hemodynamic improvement produced by PMV persists in intermediate and long-term follow-up study. The best long-term results are seen in patients with echocardiographic scores of 8 or less. When PMV produces a good immediate outcome in this group of patients, restenosis is unlikely to occur at follow-up study. Although PMV can result in a good outcome in patients with echocardiographic scores higher than 8, hemodynamic and echocardiographic restenosis is often demonstrated at follow-up study despite ongoing clinical improvement. Table 9–7 shows long-term follow-up results of patients undergoing PMV at different institutes. An estimated 12-year survival rate of 74% in a cohort of 879 patients undergoing PMV at the MGH was reported (Figure 9–5).4 Death at follow-up study was directly related to age, post-PMV pulmonary artery pressure, and pre-PMV NYHA functional class IV. In the same group of patients, the 12-year event-free survival rate (alive and free of mitral valve replacement or repair and redo PMV) was 33% (Figure 9–6). Cox regression analysis identified age (risk ratio [RR] 1.02; CI 1.01 to 1.03; p < 0.0001), pre-PMV NYHA functional class IV (RR 1.35; CI 1.00 to 1.81; p = 0.05), prior commissurotomy (RR 1.50; CI 1.16 to 1.92; p = 0.002), the echocardiographic score (RR 1.31; CI 1.02 to 1.67; p = 0.003), pre-PMV MR greater than or equal to 2+ (RR 1.56; CI 1.09 to 2.22; p = 0.02), post-PMV MR greater than or equal to 3+ (RR 3.54; CI 2.61 to 4.72; p < 0.0001), and post-PMV mean pulmonary artery pressure (RR 1.02; CI 1.01 to 1.03; p < 0.0001) as independent predictors of combined events at long-term follow-up study.
Percutaneous Balloon Valvuloplasty for Patients with Rheumatic Mitral and Tricuspid Stenosis
9.1 Patient Selection
Current Indication
Class
Level of Evidence
Symptomatic patients (NYHA functional Class II, III, or IV), moderate or severe mitral stenosis (area <1.5 cm2), and valve morphology favorable for percutaneous balloon valvuloplasty in the absence of left atrial thrombus or moderate to severe mitral regurgitation (MR).
I
Grade A
Asymptomatic patients with moderate or severe mitral stenosis (area <1.5 cm2) and valve morphology favorable for percutaneous balloon valvuloplasty who have pulmonary hypertension (pulmonary artery systolic pressure >50 mmHg at rest or 60 mmHg with exercise) in the absence of left atrial thrombus or moderate to severe MR.
IIa
Grade C
Patients with NYHA functional Class III-IV, moderate or severe mitral stenosis (area <1.5 cm2), and a nonpliable calcified valve who are at high risk for surgery in the absence of left atrial thrombus or moderate to severe MR.
IIa
Grade B
Asymptomatic patients with moderate or severe mitral stenosis (area <1.5 cm2) and valve morphology favorable for percutaneous balloon valvuloplasty who have new onset of atrial fibrillation in the absence of left atrial thrombus or moderate to severe MR.
IIb
Grade B
Patients in NYHA functional Class III-IV who have moderate or severe mitral stenosis (area <1.5 cm2), and a nonpliable calcified valve who are low-risk candidates for surgery.
IIb
Grade C
Patients with mild mitral stenosis.
III
Grade C
9.2 Technique of Percutaneous Mitral Balloon Commissurotomy
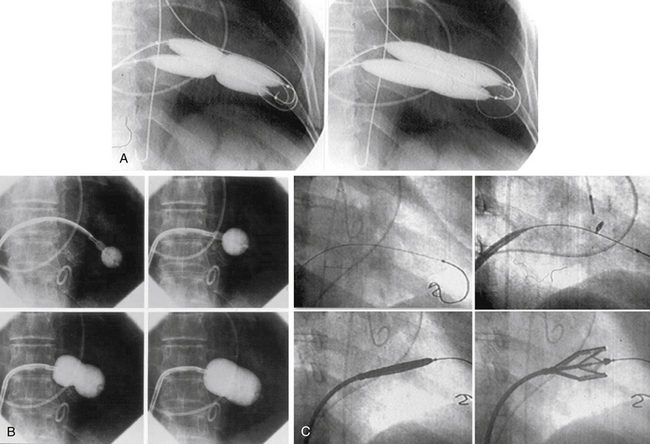
A, The double balloon technique; B, the Inoue technique; and C, the metallic valvulotome.
The Antegrade Double Balloon Technique
The Inoue Technique
![]() During inflation of the balloon an indentation should be seen in its midportion. The catheter is withdrawn into the left atrium, and the mitral gradient and cardiac output are measured. If further dilations are required the stylet is introduced again and the sequence of steps described above is repeated at a larger balloon volume. After each dilation its effect should be assessed by pressure measurement, cardiac output, auscultation, and 2D echocardiography. If MR occurs, further dilation of the valve should not be performed.
During inflation of the balloon an indentation should be seen in its midportion. The catheter is withdrawn into the left atrium, and the mitral gradient and cardiac output are measured. If further dilations are required the stylet is introduced again and the sequence of steps described above is repeated at a larger balloon volume. After each dilation its effect should be assessed by pressure measurement, cardiac output, auscultation, and 2D echocardiography. If MR occurs, further dilation of the valve should not be performed.
Mechanism of Percutaneous Mitral Balloon Commissurotomy
Immediate Outcome
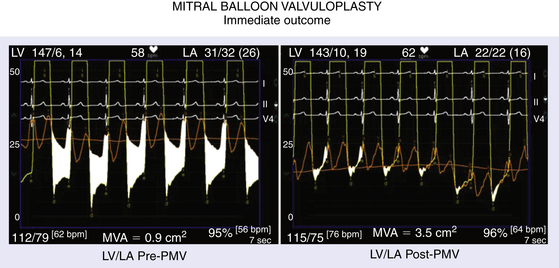
9.3 Predictors of Increase in Mitral Valve Area and Procedural Success
The Echocardiographic Score
![]() Among the four components of the echocardiographic score, valve leaflets thickening and subvalvular disease correlate the best with the increase in MVA produced by PMV.37 Therefore suboptimal results with PMV are more likely to occur in patients with valves that are more rigid and more thickened and in those patients with more subvalvular fibrosis and calcification.
Among the four components of the echocardiographic score, valve leaflets thickening and subvalvular disease correlate the best with the increase in MVA produced by PMV.37 Therefore suboptimal results with PMV are more likely to occur in patients with valves that are more rigid and more thickened and in those patients with more subvalvular fibrosis and calcification.
Balloon Size and Effective Balloon Dilating Area
Mitral Valve Calcification
Previous Surgical Commissurotomy
Age
Atrial Fibrillation
Mitral Regurgitation Before Percutaneous Mitral Balloon Commissurotomy
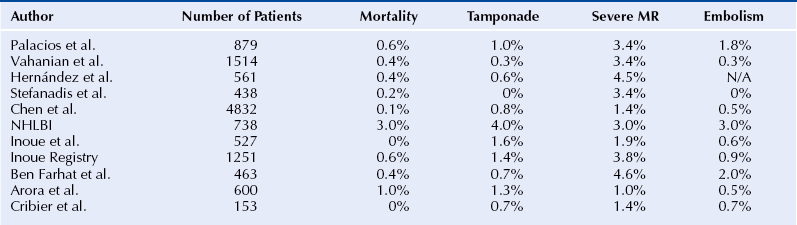
9.4 Complications
I-II
Valvular thickening (score each leaflet separately)
Leaflet near normal (4-5 mm) or with only a thick segment
Leaflet fibrotic and/or calcified evenly; no thin areas
Leaflet fibrotic and/or calcified with uneven distribution; thinner segments are mildly thickened (5-8 mm)
Leaflet fibrotic and/or calcified with uneven distribution; thinner segments are near normal (4-5 mm)
III
Commissural calcification
Fibrosis and/or calcium in only one commissure
Both commissures mildly affected
Calcium in both commissures, one markedly affected
Calcium in both commissures, both markedly affected
IV
Subvalvular disease
Minimal thickening of chordal structures just below the valve
Thickening of chordae extending to one third of chordal length
Thickening to the distal third of the chordae
Extensive thickening and shortening of all chordae extending down to papillary muscle
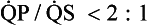 ) and clinically well tolerated. In the series from MGH there is one patient in whom the atrial shunt remained hemodynamically significant at follow-up study. This patient underwent percutaneous transcatheter closure of her iatrogenic residual atrial defect with a clamshell device. Desideri et al.45 reported atrial shunting determined by color flow transthoracic echocardiography in 61% of 57 patients immediately after PMV. The shunt persisted in 30% of patients at 19 ± 6 (range 9 to 33) months’ follow-up examination. They identified the magnitude of the post-PMV atrial shunt (
) and clinically well tolerated. In the series from MGH there is one patient in whom the atrial shunt remained hemodynamically significant at follow-up study. This patient underwent percutaneous transcatheter closure of her iatrogenic residual atrial defect with a clamshell device. Desideri et al.45 reported atrial shunting determined by color flow transthoracic echocardiography in 61% of 57 patients immediately after PMV. The shunt persisted in 30% of patients at 19 ± 6 (range 9 to 33) months’ follow-up examination. They identified the magnitude of the post-PMV atrial shunt (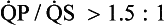 ), use of bifoil balloon (2 balloons on 1 shaft), and smaller post-PMV MVA as independent predictors of the persistence of atrial shunt at long-term follow-up study. Together with the authors’ results, these findings suggest that those patients with persistently elevated left atrial pressures resulting from either suboptimal PMV or poor diastolic compliance after PMV are more likely to experience persistent left-to-right shunting.
), use of bifoil balloon (2 balloons on 1 shaft), and smaller post-PMV MVA as independent predictors of the persistence of atrial shunt at long-term follow-up study. Together with the authors’ results, these findings suggest that those patients with persistently elevated left atrial pressures resulting from either suboptimal PMV or poor diastolic compliance after PMV are more likely to experience persistent left-to-right shunting.
9.5 Clinical Follow-Up Studies
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine