Fig. 24.1
Different ductal morphology: The ducts in pulmonary atresia can be in various morphologic forms. Type I duct, usual form: (a) The duct arises from the junction of the arch to descending aorta and courses anteriorly to insert in confluence of pulmonary arteries. Type II duct, vertical form: (b) The duct arises proximally from the undersurface of the aortic arch and courses vertically down to the confluence. Type III, tortuous form: (c) In this commonest morphological form, the duct takes a C- or S-shaped bend before inserting in the confluence. Type IV, contralateral form: (d) The duct arises opposite to the side of the aortic arch from either the contralateral innominate artery or subclavian artery. Type V, bilateral form: (e) Rarely, ducts can be bilateral, each duct will insert into ipsilateral pulmonary artery. In most of these patients, the pulmonary arteries are not confluent and are separated from each other
24.2.2 Pathophysiology
Early neonatal withdrawal of prostaglandins leads to swelling of the ductal intimal cushions, ductal constriction, and obliteration of the lumen. Functional closure of duct occurs in first 2–3 days; anatomical closure with fibrous tissue occurs later. Duct closure in pulmonary atresia results in severe hypoxia. In a few patients, ducts remain patent for a few weeks or a few months to maintain pulmonary circulation.
24.2.3 Different Anatomical Lesions
Congenital heart lesions with critically reduced pulmonary blood flows in neonatal period that depend on the duct patency to maintain pulmonary circulation can be grouped as follows [1].
24.2.3.1 Group A: Pulmonary Atresia in Biventricular Hearts
This group includes pulmonary atresia associated with tetralogy of Fallot (TOF), double outlet right ventricle, and transposition of great arteries. The anatomy is suited for a later biventricular repair with extra cardiac valve homograft or xenograft conduits.
24.2.3.2 Group B: Pulmonary Atresia in Univentricular Circulation
This group includes pulmonary atresia associated with single ventricles, unbalanced atrioventricular canals, double outlet right ventricle with non-routable ventricular septal defects, pulmonary atresia with right ventricle-dependent coronary circulation, and complex heterotaxies. They are palliated later by bidirectional Glenn and Fontan surgeries.
24.2.3.3 Group C: Transient Inadequacy of Pulmonary Circulation
(i)
Neonates having pulmonary atresia with intact ventricular septum and critical pulmonary stenosis continue to have inadequate antegrade pulmonary flows even after a successful neonatal pulmonary valvotomy for a few weeks to months
(ii)
Functional pulmonary atresia occurs before regression of high fetal pulmonary vascular resistance in neonates with severe forms of Ebstein’s anomaly, right ventricular cardiomyopathy, Uhl’s anomaly, and tricuspid valve dysplasia with regurgitation. In both of these groups, longer ductal patency is needed.
24.3 Clinical Scenarios
A few case studies highlight the different presentations of the various groups.
Case Study 1
After a fetal diagnosis of TOF with pulmonary atresia in the 6th month of gestation, a 3.2 kg baby was electively admitted after birth for observation in neonatal unit. His echocardiogram confirmed the fetal diagnosis, closing vertical duct and confluent pulmonary arteries measuring 4 mm each. After 36 h, prostaglandin E1 (PGE1) infusion was started for hypoxia. After discussion with cardiac surgeons, an elective DS with a 4 mm coronary stent was done on the fourth postnatal day with a coronary guide catheter advanced through right ventricle into the aortic arch from right femoral venous access. The acute angulation of the vertical duct from the undersurface of the aortic arch did not permit wiring the duct from femoral artery (Fig. 24.2). He later underwent elective conduit repair at 8 months of age.
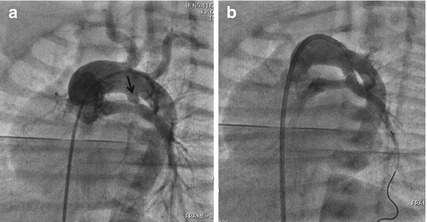

Fig. 24.2
Aortogram in shallow left anterior oblique view with a pigtail catheter advanced from femoral vein through the right ventricle into the left aortic arch (a) shows a vertical duct (arrow) arising from undersurface of the aortic arch opposite to the right innominate artery. In such cases, advancing a guidewire into distal branch of the left pulmonary artery and DS is done more easily (b) from favorable angle through the transvenous route
Case Study 2
A 10-day-old neonate weighing 2.2 kg with severe cyanosis was diagnosed as TOF, pulmonary atresia and small confluent pulmonary arteries measuring 3 mm each. Shunt surgery in small pulmonary arteries and low body weight carries high risks. DS was performed with 3.5 mm coronary stent through femoral arterial access (Fig. 24.3). Elective conduit repair was done at 1 year of age.
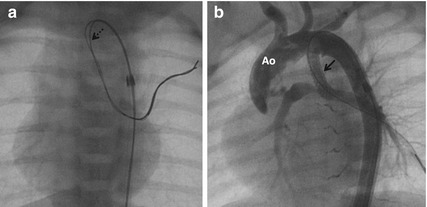

Fig. 24.3
Through a 4 F long sheath, a coronary guidewire was advanced (a) through a vertical duct. An additional buddy wire (dotted arrow) was advanced to facilitate the passage of the stent through the acute angulation of vertical duct. (b) After stenting, aortogram in shallow left anterior oblique view confirmed the stenting (arrow) of the entire length of the duct. Ao aorta
Case Study 3
A 2.8 kg neonate antenatally diagnosed with Ebstein’s anomaly of tricuspid valve presented with severe cyanosis after birth needing mechanical ventilation. There was functional pulmonary atresia, no significant antegrade pulmonary blood flows, and right aortic arch. After stabilizing with PGE1, he was weaned off the ventilator. His continued dependence on PGE1 warranted DS with a 4 mm coronary stent on the 14th postnatal day (Fig. 24.4). The improving right ventricular function normalized antegrade pulmonary blood flows after 2 months. The stent was patent for 7 months and his oxygen saturations were above 95 % throughout his childhood.
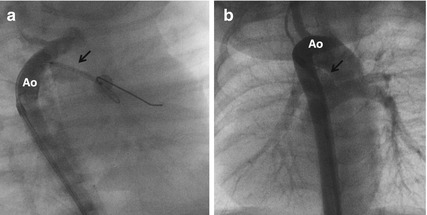

Fig. 24.4
Angiogram through a 4 F long sheath (a) in shallow right anterior oblique projection was done to check the position of a 4 mm stent (arrow) placed on a coronary guidewire through the duct advanced into the left pulmonary artery. After stenting, a repeat aortogram (b) showed good filling of the pulmonary arteries through the stented duct (arrow)
Case Study 4
A 10-day-old neonate had critical valvar pulmonary stenosis with pulmonary annulus measuring 7 mm, right to left shunt through the foramen ovale, and hypoplastic right ventricle with tricuspid valve Z-score of −2. Severe hypoxia persisted after balloon pulmonary valvotomy, even though the right ventricular pressures are reduced from 120 to 45 mmHg. His closing ductus was stented through a guide catheter advanced through the venous end into the main pulmonary artery (Fig. 24.5). His ductal stent remained patent for 1 year.
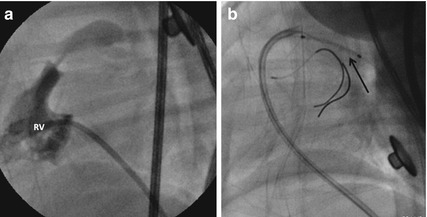

Fig. 24.5
Right ventricular (RV) angiogram in lateral view (a) demonstrates thick pulmonary valve with a narrow contrast jet into the pulmonary artery indicating severe stenosis which was dilated with a balloon. The persistent hypoxia due to inadequate antegrade pulmonary flows was an indication for ductal stent (arrow) done through a guide catheter (b) introduced from femoral vein into the pulmonary artery. A coronary guidewire was advanced from the guide catheter into the right pulmonary artery to guide the proximal extent of the ductal stent
Case Study 5
Pulmonary atresia with intact ventricular septum was diagnosed in a 4-day-old neonate with severe hypoxia. The tricuspid valve Z-score of −2 was favoring a decision for balloon pulmonary valvotomy. However, there were extensive myocardial sinusoids communicating freely with the coronary arteries and right ventricle-dependent coronary circulation (Fig. 24.6). DS with a 4 mm coronary stent was done from the femoral artery.
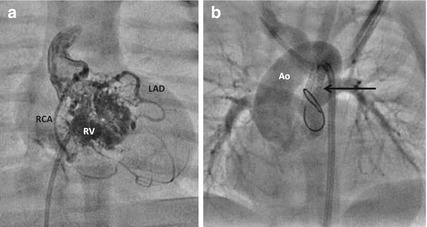

Fig. 24.6
Right ventricular angiogram (a) shows a hypoplastic right ventricle (RV) filling multiple sinusoids which fill the right coronary artery (RCA) and left anterior descending interventricular artery (LAD) indicative of right ventricle-dependent coronary circulation (RVDCC). This precludes decompression of the right ventricle with a pulmonary valvotomy. After DS, aortogram from the femoral arterial access (b) fills the well-formed pulmonary sinuses and pulmonary arteries through the stented duct (arrow)
Case Study 6
A 45-day-old infant with single ventricle, pulmonary atresia, common atrioventricular valve with no regurgitation, presented with severe hypoxia. A long duct from the contralateral right subclavian artery was very narrow at the pulmonary end. An elective DS with a 3.5 mm stent maintained adequate oxygenation till his bidirectional Glenn surgery at 1 year of age (Fig. 24.7).
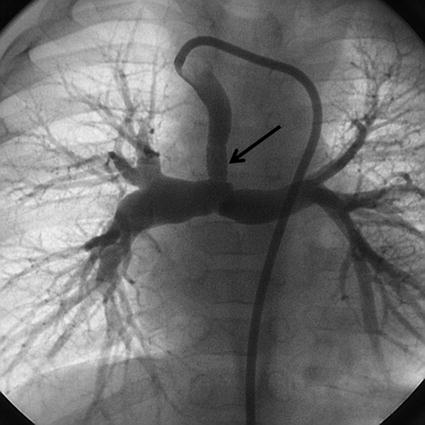

Fig. 24.7
Injection through a guide catheter passed from the left aortic arch into the right subclavian artery shows the long contralateral duct which inserts into the confluence. The narrowing in the distal insertion of the duct was stented (arrow) with a 3.5 mm stent
24.4 Indications and Patients Selection
24.4.1 Group A: Pulmonary Atresia with Biventricular Physiology
As conduit repair is deferred beyond infancy in order to place a larger conduit, a longer initial neonatal palliation is desired. In comparison with surgical shunts with 3.5 mm or 4 mm grafts, DS offers a palliation lasting only for 6–12 months. In this group, DS is only indicated in:
(i)
High-risk surgical candidates: low birth weight below 2.5 kg, syndromes like trisomy 21, comorbidities like bronchopneumonia, lung disease of prematurity, and other organ malformations.
(ii)




Small pulmonary arteries. Surgical shunts on very small pulmonary arteries are complicated by frequent shunt site narrowing. The distal hilar pulmonary artery narrowing after shunt is difficult to repair during the corrective surgery. In contrast narrowing of confluence after DS is easily approachable in corrective surgery.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


