Chapter 6 Patients’ problems, physiotherapy management and outcome measures
INTRODUCTION
This chapter discusses the problems commonly encountered by the physiotherapist when working with patients who have respiratory or cardiovascular dysfunction. The patient problems identified in this chapter are those developed following assessment of the patient and are most likely to respond to physiotherapy treatment. This chapter will assist the physiotherapist to utilize clinical reasoning skills by linking and interpreting information (subjective and objective findings) to develop an analysis that is based on the patient’s problems (Chapter 1). The presence of pathology affecting the respiratory and cardiovascular systems affects normal physiological functioning and the signs and symptoms produced are the clinical manifestations of this pathophysiology. The physiotherapist therefore requires a thorough knowledge of normal physiology as well as the pathology and pathophysiology of the respiratory and cardiovascular systems. In addition, an understanding is required of the possible sequelae of the pathological process, the clinical presentations of the disorder(s), the likely impairments, activity limitations and participation restrictions, impact on quality of life (QoL) and the anticipated prognosis for the patient.
PROBLEM SOLVING
 musculoskeletal dysfunction – postural abnormalities, decreased compliance or deformity of the chest wall.
musculoskeletal dysfunction – postural abnormalities, decreased compliance or deformity of the chest wall.Case studies
CASE STUDY 6.1
A 57-year-old woman is admitted to a tertiary hospital via the emergency department.
History of presenting condition
Chronic obstructive pulmonary disease (COPD)
Gastro-oesphageal reflux disorder (GORD)
Osteoarthritis affecting both knees.
Angiotensin-converting enzyme (ACE) inhibitor for hypertension
Proton pump inhibitor for GORD
Statin for raised cholesterol.
Lives at home with her husband but is finding it increasingly difficult to manage
Pulmonary function tests were performed at a recent clinic visit at a time when her condition was stable Table 6.1.
Current medical investigations
Respiratory rate 28 breaths/min
Sputum microculture result Pseudomonas aeruginosa.
Physiotherapy subjective examination
Smoked 25 cigarettes per day for 30 years (ceased 10 years ago).
Physiotherapy objective examination
Patient appears thin and frail
Barrel-shaped chest with increased anteroposterior diameter and thoracic kyphosis
Increased use of abdominal muscles during expiration
Using pursed-lip breathing (PLB) during conversation
Chest expansion symmetrical and poor in all zones
Sputum productive of 20 ml thick, purulent mucus (P2) expectorated in past 12 hours
Cough effective and tight but having significant difficulty expectorating sputum Table 6.2.
Table 6.1 Pulmonary function tests, case study 1
| Test | Observed | Predicted |
|---|---|---|
| FVC (l) | 2.76 | 2.90 |
| FEV1 (l) | 1.04 | 2.30 |
| FEV1/FVC (%) | 38.00 | 77.00 |
| FRC (l) | 4.67 | 2.62 |
| RV (l) | 3.24 | 1.96 |
| TLC (l) | 6.16 | 5.02 |
| TLCO (mmol/min/kPa) | 4.16 | 7.59 |
FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; FRC, functional residual capacity; RV, residual volume; TLC, total lung capacity; TLCO, transfer factor of the lung for carbon monoxide
Table 6.2 Analysis of problems from case study 1
Note: respiratory muscle dysfunction may be present (clinical features include orthopnoea, CO2 retention and altered breathing pattern) and further assessment may be warranted
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; TLC, total lung capacity; FRC, functional residual capacity; PLB, pursed-lip breathing; TLCO, transfer factor of the lung for carbon monoxide; GORD, gastro-oesophageal reflux disorder; 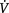 /
/ , ventilation/perfusion ratio; VT, tidal volume
, ventilation/perfusion ratio; VT, tidal volume
CASE STUDY 6.2
History of presenting condition
Sudden onset of abdominal pain and vomiting.
Diabetes mellitus controlled with diet
Ischaemic heart disease (IHD).
Home medications: aspirin, nitrate, ACE inhibitor, β−adrenoreceptor blocker, statin and diuretic
Lives at home with wife and is independent
Smoked 20 cigarettes a day for 40 years (ceased 2 years ago).
Current medical investigations
Respiratory rate 28 breaths/min
Nasogastric tube aspirate 300 ml over 24 hours.
Urine output 1700 ml over 24 hours
White blood cell count (WCC) 14 × 109/l
Physiotherapy subjective examination
Reports that he has coughed very little overnight and today
No sputum produced either overnight or today
Only ambulating with assistance, as a result of persistent nausea
Reports that he forgets to use the PCA.
Physiotherapy objective examination
Appears ill and is pale and clammy
Chest expansion poor lower zones: right < left
Abdominal splinting on inspiration
Cough is painful, weak, moist and ineffective
Auscultation reveals decreased breath sounds left lower lobe, right middle and lower lobes
Nil calf tenderness, warmth or redness
Table 6.3 Analysis of problems from case study 2
| Current cardiopulmonary problems | Evidence for each problem based on clinical features | Most likely pathophysiological basis for each problem |
|---|---|---|
| Reduced lung volume | Atelectasis resulting from marked decrease in FRC, postoperative diaphragmatic dysfunction, reduced function of surfactant, airway obstruction from mucus plugging and abdominal incisional pain | |
| Impaired gas exchange | Acute hypoxaemia: PaO2 7.8 kPa (59 mmHg) and SaO2 91% on room air | Mixed causes for hypoxaemia including 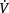 / / mismatch caused by a decrease in FRC mismatch caused by a decrease in FRC |
| Impaired airway clearance | Patient is febrile, elevated WCC, moist cough | Possible colonization of sputum, possible systemic dehydration, impaired MCC from opioid, ineffective cough |
| Considerations for treatment | Reduced mobility further increases risk factors for PPC, a reduction of oxygen transport and risk of a deep vein thrombosis – mobilization of patient is a major goal of treatment | |
FRC, functional residual capacity; MCC, mucociliary clearance; WCC, white blood cell count; PPC, postoperative pulmonary complication; IHD, ischaemic heart disease; PCA, patient-controlled analgesia, 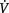 /
/ , ventilation/perfusion ratio; PaO2, partial pressure of oxygen in arterial blood; SaO2, arterial oxygen saturation
, ventilation/perfusion ratio; PaO2, partial pressure of oxygen in arterial blood; SaO2, arterial oxygen saturation
The problem-solving approach to determining patient management not only assists with the identification of existing problems but also enables recognition of potential patient problems. For example, a high-risk surgical patient will develop reduced lung volume and has the added potential to develop problems of impaired airway clearance but if active treatment, such as early ambulation, is started during the at-risk period, these problems may be prevented. Some problems are not amenable to physiotherapy intervention or physiotherapy intervention may be detrimental.
The selection and use of appropriate outcome measures are fundamental to the evaluation of physiotherapy intervention. Healthcare fundholders increasingly require data demonstrating the effects of physiotherapy intervention, using instruments that are reliable and valid. Other parties requiring outcome data include the patient, the patient’s relatives and caregivers, employers of physiotherapists, clinicians, patient support groups and associations of patients with particular conditions (e.g. cystic fibrosis, heart failure), members of other healthcare professions and insurers. Thus, when selecting which outcome data to monitor it is important to consider the relevant stakeholders.
PROBLEM – IMPAIRED AIRWAY CLEARANCE
Normal airway clearance depends upon two mechanisms – mucociliary clearance (MCC) and effective cough. Alveolar clearance may also contribute to the clearance of secretions from the peripheral airways (Houtmeyers et al 1999a).
Secretions and debris in the small airways are transported toward the large airways by the mucociliary blanket or escalator and eventually swallowed or cleared by a cough. The mucociliary escalator consists of cilia and a mucus layer. Impurities are caught in the mucus layer and the cilia beat synchronously to move the mucus towards the upper airway. In health, airway mucus is composed mostly of water and the daily volume of mucus in a healthy adult is up to 100 ml (Clarke 1990).
While mucociliary transport is the major mechanism for clearing secretions in healthy subjects, cough is an important mechanism, especially in people with lung disease. The effectiveness of a cough is related to the volume and viscosity of secretions and the velocity of airflow through the airway lumen. An effective cough requires a high flow rate and a small cross-sectional area of the airway. Dynamic compression of the airways starts downstream from the equal pressure point (Chapter 5) where intraluminal and extraluminal pressures around the bronchial wall are equal (Irwin & Widdicombe 2000). This compression will increase airflow velocity by decreasing the cross-sectional diameter of the airways.
Vigorous coughing can cause a number of adverse effects including abnormal cardiovascular responses (e.g. systemic hypotension and hypertension, rhythm disturbances), abnormalities of the genitourinary tract (e.g. urinary incontinence), gastrointestinal symptoms (e.g. gastro-oesophageal reflux, inguinal hernia), musculoskeletal problems (e.g. rupture of rectus abdominis, rib fractures), neurologic features (e.g. cough syncope, headache, stroke, seizures) and respiratory complications (e.g. airflow limitation, laryngeal trauma, pneumothorax, tracheobronchial trauma). These effects are largely due to the high intrathoracic pressures and expiratory velocities associated with vigorous coughing (Irwin & Widdicombe 2000).
Abnormalities in the normal airway clearance system (i.e. MCC and cough) will result in an accumulation of secretions causing airway obstruction and possibly lead to atelectasis. The subsequent inhomogeneity of ventilation may adversely affect gas exchange. Airway obstruction and the presence of excess secretions also increase the risk of infection. Inflammatory responses to infection cause the release of chemical mediators such as proteases and elastases that can destroy the airway epithelium. This leads to unstable, overcompliant airways that contribute to impaired airway clearance (Barker 2002).
Table 6.4 lists the pathophysiological basis of impaired airway clearance and includes clinical examples (Clarke 1990, Houtmeyers et al 1999a, Irwin & Widdicombe 2000).
Table 6.4 Pathological basis of impaired airway clearance and clinical examples
| Pathophysiological basis | Comment and clinical examples | |
|---|---|---|
| Increased or altered composition of mucus | Bronchiectasis, chronic bronchitis, cystic fibrosis, asthma, pneumonia Presence of an artificial airway increases mucus secretion Changes viscosity and increases amount of secretions, thereby slowing MCC Leads to viscous secretions which are difficult to mobilize and expectorate May occur postoperatively if fluid restriction imposed Excess fluid loss due to prolonged very high respiratory rate | |
| Abnormalities in cilial structure or function | Primary ciliary dyskinesia Damage to ciliated epithelium from excessive endotracheal suctioning | |
| Impaired MCC | Rate of MCC decreases with age Decreases MCC e.g. Tobacco smoke, NOx – may decrease MCC Some general anaesthetics and narcotics depress MCC May cause a loss of ciliated epithelium causing mucus retention and slowing MCC Slows MCC Coughing and expectoration may be avoided due to embarrassment | |
| Abnormal cough reflex | 1. Decreased | Decreased level of consciousness, general anaesthesia, narcotic analgesics Inhibition due to pain, e.g. postoperatively, chest trauma, pleurisy Damage to vagal or glossopharyngeal nerves Laryngectomy Paralysed vocal cords Denervated lungs (heart-lung or lung transplantation) |
| 2. Increased | Bronchial hyperreactivity Poorly controlled asthma Viral infections may increase sensitivity | |
| Ineffective cough due to the inability to generate sufficient expiratory flow | Severe reduction in VC Respiratory muscle weakness Airflow limitation may cause cough to be weak and/or ineffective Decreased airflow through dilated bronchiectatic airways | |
| Abnormal cough | Stimulates the cough reflex May lead to chronic cough and microaspirations of gastric contents |
MCC, mucociliary clearance; NOx nitrogen oxides; VC, vital capacity; GORD, gastro-oesophageal reflux disorder
Special case – postoperative patient with impaired airway clearance
Many factors either present preoperatively or arising in the perior postoperative period increase mucus secretion and/or impair MCC and may be responsible for the development of PPC. It is therefore important for the physiotherapist to identify patients who have an increased risk of developing PPCs (Chapter 12).
Clinical features
The clinical features of impaired airway clearance are usually those resulting from excess or retained secretions. The history of usual daily sputum production obtained from the patient may reveal a chronic productive cough. Changes in the normal pattern of sputum production, such as an increase in the amount or a change in the colour or consistency of the sputum, are likely. Some patients report difficulty expectorating secretions. Further questioning may reveal an increase in the number of chest infections or hospitalizations for their illness compared with previous years. Patients with chronic lung disease may report signs of stress incontinence on coughing.
Examination of the patient may reveal an altered breathing pattern due to increased work of breathing (WOB). The presence of infection may produce fever and tachycardia. When the secretions cause marked airflow limitation, wheezing may be audible (see Problem – airflow limitation). Auscultatory findings include diminished or absent breath sounds, bronchial breath sounds, crackles or wheezes. The cough may be moist or dry and hacking, effective and productive or ineffective and weak. Some patients have a paroxysmal cough with associated adverse effects such as dizziness, syncope or exhaustion. The examination of any sputum expectorated may reveal an increase in the volume or weight compared with the patient’s normal expectorant. The colour of the sputum may have changed to yellow, green or brown and there may be blood present (haemoptysis). Also the consistency of the sputum may have altered and microculture may reveal colonization (e.g. with bacteria) (Chapter 1).
The clinical features of impaired airway clearance in the postoperative patient may include an increased volume of sputum expectorated compared with the patient’s usual expectorant; a weak, ineffective moist cough; possible bacterial contamination of expectorated sputum; fever; and chest radiographic changes consistent with atelectasis or pneumonia (Chapter 12).
Physiotherapy management
Airway clearance techniques comprise a range of physiotherapy interventions used for the management of impaired airway clearance (Chapter 5). These techniques aim to promote clearance of excessive secretions from the distal airways and thereby prevent the consequences of obstruction and thus improve ventilation homogeneity and gas exchange. Airway clearance techniques may incorporate positive pressure or oscillation applied at the mouth or chest wall (manual or mechanical) and/or breathing strategies to aid the movement of secretions to the central airways. From the central airways, forced expiratory manoeuvres such as coughing or huffing are used to facilitate expectoration. Such manoeuvres aim to use high expiratory flow rates to shear secretions from the airway walls.
The physiotherapy management of impaired airway clearance is influenced by the underlying cause and acuity of the patient’s condition. For patients with chronic hypersecretory lung disease who regularly produce excess bronchial secretions, the use of daily airway clearance techniques is recommended (Jones & Rowe 1998, van der Schans et al 2000). The rationale for daily treatment is to reduce stagnation of secretions in an attempt to avoid contamination with pathogens and thereby reduce the destruction of airway walls caused by the inflammatory response. This may slow the cycle of progressive tissue damage. The physiotherapist’s role in this case is to prescribe and teach a daily airway clearance regimen that is individually tailored and acceptable to the patient. Factors to be considered when choosing an airway clearance technique include:
A number of additional measures are available that have been shown to enhance or improve airway clearance. Table 6.5 outlines these measures (Conway et al 1992, Elkins et al 2006, Houtmeyers et al 1999b, Jones et al 2003, Wark et al 2005, Wills & Greenstone 2006).
Table 6.5 Additional measures to enhance airway clearance
| Measure | Examples and uses |
|---|---|
| Humidification | |
| Nebulization | MCC may be improved by hypertonic saline, amiloride, recombinant human deoxyribonuclease and β-adrenergic agonists |
| Analgesia | Patients in whom pain is inhibiting an effective cough |
| Physical activity | Increased respiratory rate and VT increase expiratory flow rates and sputum clearance |
NIV, non-invasive ventilation; MCC, mucociliary clearance; VT, tidal volume
In the postoperative patient it is essential to establish whether the patient has excess secretions and whether they have difficulty managing their own airway clearance. This is one of the factors influencing the risk of the patient developing PPCs. The techniques to assist sputum clearance in the postoperative patient aim to increase alveolar ventilation and expiratory flow rates using, for example, upright positioning and ambulation at an adequate intensity with encouragement to take deep breaths. Expectoration of secretions can be facilitated by supported coughing or huffing (Chapter 12). If the patient has large amounts of secretions, techniques such as the active cycle of breathing techniques (ACBT) and vibrations may be used.
For patients who are reluctant or unable to cough, a spontaneous cough may be elicited by physical activity or a change of position. The cough reflex may be elicited using a tracheal rub or suctioning. Strengthening of the abdominal muscles and assisted cough techniques (e.g. abdominal support with an upward pressure) or a mechanical insufflation- exsufflation device may be helpful for patients with impaired cough due to weakness of the abdominal muscles (Chapter 16). For the intubated and ventilated patient, improved alveolar ventilation and increased expiratory flow rates can be achieved by positioning and manual hyperinflation, and secretions cleared by suctioning (Chapter 8).
Outcome measures
Short-term outcomes can be monitored by a change in sputum expectorated, as measured by weight, volume or rate of expectoration. Ease of sputum expectoration can be measured using a categorical scale or visual analogue scale (VAS). In acute conditions, chest radiographs and auscultatory findings may provide evidence for a change in the patient’s condition. Radio-aerosol clearance may be used as an outcome measure in studies of airway clearance techniques (van der Schans et al 1999).
Long-term outcomes in patients with hypersecretory lung disease may be assessed by the number of exacerbations, courses of antibiotics, hospitalizations and days lost from work/study per year. Quality of life scales, such as the St George’s Respiratory Disease Questionnaire (SGRQ), include a section that quantifies symptoms of cough and sputum (Jones et al 1992). Pulmonary function, in particular spirometry, has been used to evaluate the effects of airway clearance techniques but may be relatively insensitive to the intervention (van der Schans et al 1999).
PROBLEM – DYSPNOEA
Dyspnoea is the term generally applied to the sensations experienced by individuals complaining of unpleasant or uncomfortable respiration (Ambrosino & Scano 2001). In clinical practice, the terms breathlessness and dyspnoea are used interchangeably. However ‘breathlessness’ is one of many descriptors used by patients to convey their experience of dyspnoea. Other common terms used by patients suggest unrewarded inspiration (i.e. ‘can’t get the air in’) and chest tightness. It is possible these different descriptors originate from different pathological processes. For example, individuals with COPD frequently use terms that reflect an increase in the effort of breathing or WOB (Scano et al 2005).
Many healthy individuals become aware of their breathing when exercising at a moderate or high intensity and report that their breathing is rapid and that they are puffing. These changes in breathing reflect the increased ventilation required during exercise and are appropriate for the situation. In contrast, individuals with respiratory or cardiovascular disease may become aware of unpleasant breathing sensations at very low levels of physical activity and even at rest or in response to emotional or stressful situations. In such situations the appropriate term for these respiratory sensations is dyspnoea. Dyspnoea is not tachypnoea, hyperventilation or hyperpnoea. These three terms all describe ventilation in response to different stimuli and may represent normal physiological responses. Although hypoxaemia and hypercapnia increase ventilatory response, the severity of hypoxaemia and hypercapnia are not directly linked to the perception of dyspnoea. The sensation of dyspnoea appears to originate with the activation of sensory systems within the lung, chest wall and respiratory muscles that give rise to an awareness of breathing discomfort (American Thoracic Society (ATS) 1999, Schwartzstein & Parker 2006).
The sensation of dyspnoea is influenced by many factors including the patient’s psychological status and their experience and memory. The presence of fear, anxiety, depression and anger heighten the perception of dyspnoea (ATS 1999). In some patients, dyspnoea may be perceived as life threatening. A patient’s ability to describe and quantify the unpleasant sensation of dyspnoea is also very variable. This is not unlike the variability seen when patients report pain. These factors may in part explain why the intensity of dyspnoea for a given level of impairment in lung function, or exercise capacity, can vary greatly among individuals.
Although it is generally contended that dyspnoea arises as a consequence of multiple complex and varied interactions, the precise mechanisms responsible for the sensation of dyspnoea are poorly understood and the management of dyspnoea poses considerable difficulties. Clinically, dyspnoea results from several different pathophysiological mechanisms and in some patients more than one mechanism will be responsible (Table 6.6) (ATS 1999, Scano et al 2005, Schwartzstein & Parker 2006).
Table 6.6 Pathophysiological basis for dyspnoea in respiratory and cardiovascular disease and clinical examples
| Pathophysiological basis | Clinical examples | |
|---|---|---|
| 1. Increase in elastic load due to: | a. Decrease in lung compliance | Increases the inspiratory muscle work required to overcome the elastic recoil of the lungs. Increases in 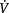 E are achieved mainly by increasing respiratory rate, e.g. ILD, breathing at low lung volumes, pulmonary congestion E are achieved mainly by increasing respiratory rate, e.g. ILD, breathing at low lung volumes, pulmonary congestionHyperinflation (e.g. COPD, cystic fibrosis, asthma) increases the WOB |
| b. Decrease in chest wall compliance and /or compliance of the abdominal compartment | Obesity, kyphoscoliosis, ankylosing spondylitis | |
| 2. Increase in airways resistance | Increases expiratory muscle work to effect airflow through narrowed airways (e.g. COPD, asthma) | |
| 3. Weakness or fatigue of the respiratory muscles | See Problem – respiratory muscle dysfunction | |
| 4. Increase in metabolic rate | Increases ventilatory requirements, e.g. fever, exercise | |
| 5. Low cardiac output / ischaemia | Inadequate cardiac output causes reflex medullary ventilatory stimulation when the oxygen supply to the exercising muscle is inadequate to meet metabolic needs, e.g. IHD, heart failure or in the presence of ventricular arrhythmias, valvular problems or cardiomyopathy | |
| 6. Blood gas abnormalities | Hypoxaemia or hypercapnia | |
| 7. Deconditioning | Lactate accumulates at low levels of exercise causing an increase in ventilation | |
| 8. Anaemia | When severe causes dyspnoea on exertion | |
| 9. Acute changes in permeability of pulmonary capillaries | Pulmonary oedema | |
| 10. Perfusion limitation | The presence of a large 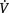 / / mismatch or shunt invariably causes dyspnoea, e.g. pulmonary embolus, pulmonary infarction, cyanotic heart disease, pulmonary congestion mismatch or shunt invariably causes dyspnoea, e.g. pulmonary embolus, pulmonary infarction, cyanotic heart disease, pulmonary congestion |
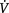 E, minute ventilation; ILD, interstitial lung disease; COPD, chronic obstructive pulmonary disease; WOB, work of breathing;
E, minute ventilation; ILD, interstitial lung disease; COPD, chronic obstructive pulmonary disease; WOB, work of breathing; 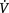 /
/ , ventilation/perfusion ratio
, ventilation/perfusion ratio
Special case – chronic lung disease
Some patients with moderate or severe disease, especially those with COPD, report marked dyspnoea when performing activities of daily living (ADL) that involve the use of the upper limbs, especially when the upper limbs are unsupported. Performing activities that involve unsupported upper limb movements leads to a loss of these arm trunk muscles as elevators of the rib cage, thereby reducing their contribution to the generation of the intrapleural pressure needed for inspiration. The breathing pattern during unsupported upper limb exercise in patients who report dyspnoea with upper limb movements is often rapid and irregular, and dyssynchronous thoraco-abdominal movements and breath holding may occur (Celli 1994). A further workload is imposed when activities involve raising the arms above the head. This arm position gives rise to the early onset of lactate accumulation in the upper limbs leading to an increase in carbon dioxide (CO2) production, which stimulates ventilation.









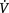 /
/ mismatch, diffusion limitation, wasted perfusion (intrapulmonary shunt) and hypoventilation
mismatch, diffusion limitation, wasted perfusion (intrapulmonary shunt) and hypoventilation CO2 and increased dead space as a fraction of VT
CO2 and increased dead space as a fraction of VT







