Overview of the pathophysiology of renal insufficiency in HFREF. (a) Organ-specific factors: Reduction in RBF and increased (renal) venous pressure, resulting in increased renal interstitial pressure (directly opposing filtration in Bowmans capsule (b)). Glomerular factors: Renal autoregulation preserves GFR, a process inhibited by RAAS inhibitors causing (pseudo) worsening renal function. Non-steroidal anti-inflammatory drugs inhibit prostaglandin synthesis, thereby impairing prostaglandin associated increase/dependent renal blood flow. Concomitant diseases have direct, but differential effect on glomerular filtration, glomerular integrity and podocyte function, as well as autoregulation. (c) Nephronic factors: the combination of increased interstitial pressure, reduced arterial perfusion, concomitant disease and therapies can cause tubular and glomerular injury. Increased renal interstitial pressure causes collapsing of renal tubules, thereby lowering GFR, and eventually leading to decreased urine output, sodium retention, and congestion. Abbreviations: ACEi, angiotensin-converting enzyme inhibitor: ARB, angiotensin II receptor blocker: FF, filtration fraction: GFR, glomerular filtration rate: MRA, mineralocorticoid receptor antagonist: NSAIDs, non-steroidal anti-inflammatory drugs: RAAS, renin–angiotensin–aldosterone system: RBF, renal blood flow. (From Damman et al. [21])
Important considerations when approaching a HFREF patient with renal dysfunction
Current situation: Hemodynamics Is the patient stable? If not, this should be the first treatment goal. Excessive congestion? Evidence of edema? Hypo or hypertensive? |
Predisposing conditions that can cause (more than expected) renal impairment Diabetes mellitus Atherosclerosis Hypertension |
Background therapy Any medical therapy that can compromise renal function? Any medical therapy that is renally cleared? What about HF therapy: what is the current type and dose of evidence based HF therapy, especially RAAS inhibitors? Use of (loop) diuretics? |
Dynamics in renal function What was the course of eGFR/serum creatinine in the past weeks/months? What was the most likely reason for the change? Any indication of organ damage? What about albuminuria (especially in hypertensives, diabetics) |
Any indication of adverse events linked to renal dysfunction? Hyperkaleamia Gout like symptoms Muscle cramps |
Hyperkalaemia
Hyperkalaemia is a frequent condition that occurs in patients with heart failure and concomitant renal dysfunction. Normally, hyperkaleamia is defined as a potassium above 5.0 mmol/L. Up to 25–30% of chronic HFREF patients may develop at some stage hyperkaleamia, which may be due to underlying conditions, such as renal dysfunction, or can occur after initiation and/or uptitration of evidence based heart failure therapies. It is therefore a very important disorder that prohibits sometimes the uptitration of RAAS-inhibitor therapy, and in specific mineralocorticoid receptor antagonists (MRA’s) that are known to elevate potassium levels. Some obvious causes of hyperkaleamia such as inadequate blood draw, use of potassium supplements or metabolic disorders should always be considered and excluded. Then, as with the current patient is the case, if no other causes can be identified, care should be taken to re-evaluate potassium levels regularly. If any further increase is observed, either MRA (or other RAAS inhibitor) should be downtitrated, or if the patient is congested, loop diuretics can be initiated, which are known to reduce serum potassium levels. |
Novel treatments to specifically lower potassium levels to allow further uptitration of evidence based therapies are now being evaluated but have not found their way to clinical practice yet. |
Worsening renal function
Changes in filtration rate occur all the time in patients with HFREF, and even with daily determination of serum creatinine it is difficult to establish true alterations in GFR. Improvements in serum creatinine and GFR will hardly alert any clinician, while certain increases in creatinine will quickly alarm many HF specialists. From epidemiological data, any increase in serum creatinine, whatever the cause, was associated with worse clinical outcomes. However, the magnitude of this excess risk (which can be minimal to substantial) depends entirely on the circumstances during which this increase developed. If the clinical status of a patient improves, but serum creatinine increases, this normally is not associated with worse outcomes. It should prompt re-evaluation after some time, but would not necessarily need any action to be taken. Similarly any modest increase in serum creatinine after RAAS-inhibitor initiation or uptitration should be expected and accepted, even in patients with already compromised kidney function. Only very large (and unexpectedly large) increases in serum creatinine should alarm the HF specialist to reduce or even stop these life saving drugs. Always check whether other factors could have been responsible for the deterioration in renal function, such as over the counter NSAIDs, antibiotics, loop diuretics, or clinical deterioration. If the patient remained stable, re-challenge with a RAAS inhibitor should be considered, possibly in lower dosages and a slower uptitration regime. In difficult cases, a consultation by nephrologist should be considered. |
In the current case, this patient does have a strikingly reduced estimated GFR (25 mL/min/1.73 m2), more than might be expected from his age, creatinine and severity of HF. In such a situation, it is important to re-evaluate findings and medical history to understand the disproportional low eGFR. It could be that the hemodynamic status of the patient is more compromised than can be seen with minimal examination and anamnesis. If this is suspected, care should be taken to get objective evidence of to support this. More importantly, not only HF induces a decline in renal function, also many comorbidities exert detrimental effects, some of which contribute to the development of HF as well. Particularly, atherosclerosis, hypertension and diabetes mellitus are associated with worse renal function and more renal function decline in non HF populations, and all are associated with the development of HF by themselves [23, 24]. What this actually means is that in some patients, before the development of (overt) HF, renal function is often already compromised [25]. In the current case, this patient was already suffering from poorly controlled diabetes with end organ damage (retinopathy and nephropathy) probably long before HF occurred after the myocardial infarction. Although this did not translate in to an elevated serum creatinine level when the infarction occurred, diabetes can cause accelerated decline in renal function, cause glomerulosclerosis and tubular injury, as well as causing nephron loss [26]. Diabetic patients are also known to have renal hyperfiltration where filtration fraction (GFR divided by renal blood flow) actually increases; which is thought to be a sign of renal compensation, but also a sign of renal end organ damage [27]. This might have been the case with the current patient when serum creatinine was still normal at the time of the coronary event. Although hyperfiltration normally doesn’t occur in hypertension, this condition is also associated with accelerated decline in renal function and loss of nephrons [28]. It is also a major risk factor for HF, either directly or through promoting cardiovascular events [29]. Controlling blood pressure and optimizing diabetic regulation are therefore important treatment targets in patients at risk of HF, but also in HF patients themselves, since this might be associated with favourable renal outcomes. Although this has not been shown in an evidence based manner, it is unlikely that pathophysiological processes associated with early renal function decline in patients without (or before) HF are either halted, attenuated or even reversed when overt HF develops. Therefore, from a renal perspective, taking care of blood pressure and especially diabetic control, should be part of the treatment of HFREF patients with renal insufficiency.
Treatment of HFREF Patients with Renal Insufficiency
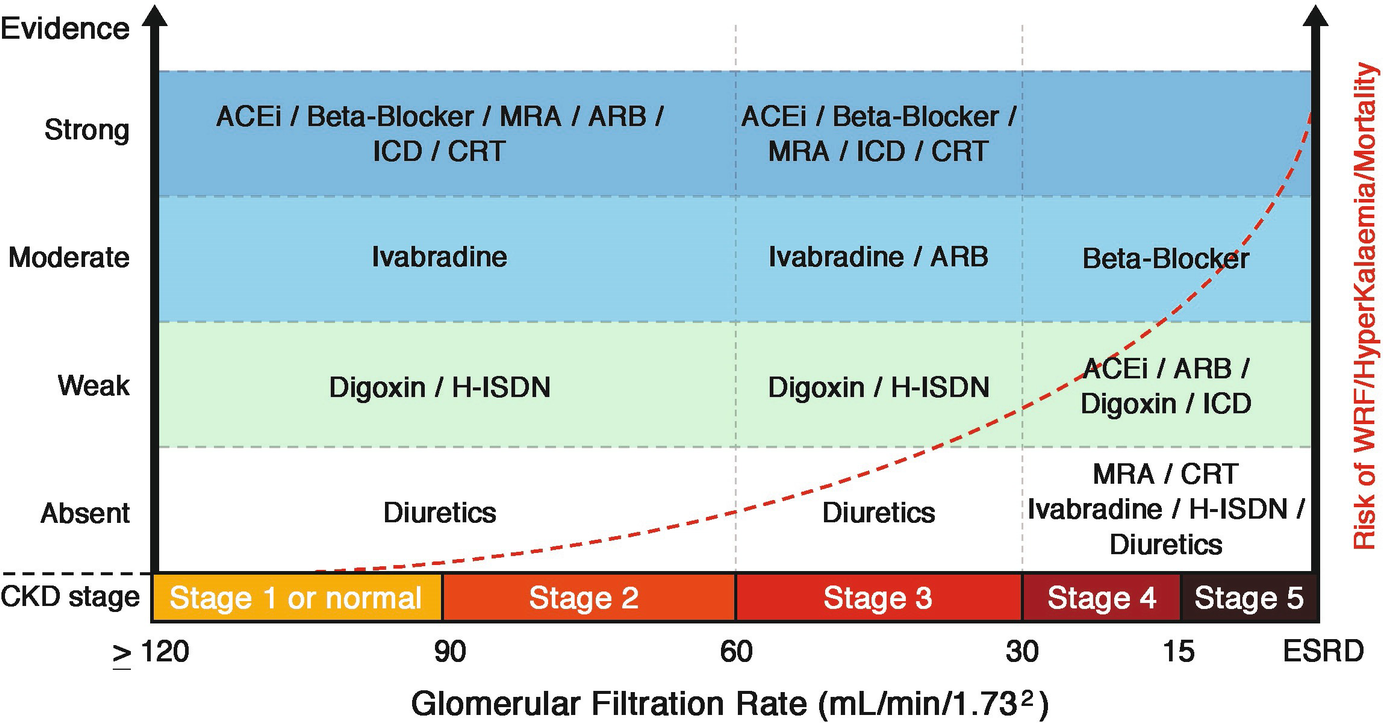
Evidence of guideline recommended treatments in HFREF according to CKD stages. Angiotensin blocker neprilysin inhibitor (ARNI) shows the same evidence as for ACEi, although only in one study. Abbreviations: ACEi: angiotensin-converting enzyme inhibitor, ARB: angiotensin II receptor blocker, CKD: Chronic kidney disease, CRT: cardiac resynchronization therapy, ESRD: End stage renal disease, GFR: glomerular filtration rate, H-ISDN: hydralazine and isosorbide-dinitrate, ICD: implantable cardioverter-defibrillator, MRA: mineralocorticoid receptor antagonist, RAAS: renin angiotensin aldosterone system. (From Damman et al. [30])

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


