Pathophysiology of Patients with Congestive Heart Failure
James B. Young
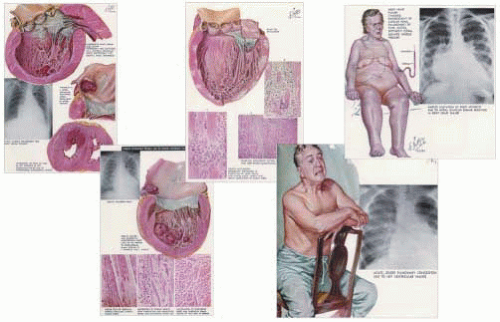
Though many clinicians believe they can easily describe a patient with heart failure, in fact, the definition of the syndrome is nebulous. Previously, congestive heart failure was thought of primarily as a dropsical or fluid retention state in a setting, generally, of hypertension, valvular heart disease, or chronic ischemic heart syndromes. Perhaps the best representation of this is the remarkable illustrations created by Dr. Frank Netter in The Ciba Collection of Medical Illustrations series (1). Figure 32.1 is a montage of his depiction of a patient with chronic congestive heart failure in comparison to an acute pulmonary edema patient. The fluid retention state is part of a syndrome where cardiomegaly is noted, pulmonary edema and mesenteric congestion is apparent, and the patient is symptomatic with profound weakness, fatigue, and dyspnea—both at exertion and at rest (orthopnea and paroxysmal nocturnal dyspnea). Acute heart failure is also often noted and generally seen in individuals with sudden pulmonary edema associated mostly with hypertensive crises, acute valvular insufficiency (sudden aortic or mitral insufficiency), or acute coronary syndromes (unstable angina and myocardial infarction). These disease states can also be blamed for subsequent ventricular hypertrophy and cardiac chamber dilation. Also displayed in the Fig. 32.1 montage is Netter’s summary of the etiologic events leading up to, and the pathophysiology of, heart failure. Early definitions of heart failure focused on the concept that hemodynamic abnormalities were largely rooted in systolic and, to a lesser extent, diastolic dysfunction rather than detrimental cardiac remodeling. A quite commonly quoted definition of heart failure previously described the state as one in which the cardiac output was inadequate to meet the metabolic demands of the body. It was felt that this led to the circulatory, and particularly renal perfusion, aberrations that prompted fluid retention, leading to congestion. Of course, this definition of heart failure is correct, however, limited in scope. Heart failure is a vastly more complex syndrome, and we now know that cardiac and circulatory failure can be seen in patients with more normal intracardiac hemodynamics and cardiac output. This has been summarized in many recent overviews (2,3,4,5,6).
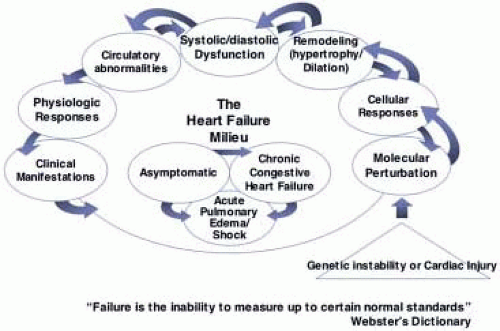
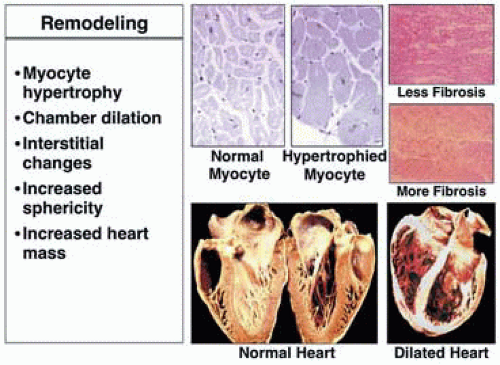
Figure 32.2 attempts to put into context the many facets of the heart failure syndrome that, perhaps, better explain its pathophysiology and define the syndrome. Primarily, heart failure should take into context the broader definition of “failure,” which is the inability to measure up to certain normal standards (Fig. 32.2). Thus, heart failure can be defined as failure of normal molecular biodynamic processes, failure of normal cellular responses to signaling hormones or other circulating substances, and failure of the heart with respect to proper filling and emptying. Failure, too, can be defined by circulatory flow perturbation, sometimes so subtle that it cannot be objectively measured or easily studied. As Fig. 32.2 suggests, one sort of injury or disease induction process is noted, with these changes then intertwining with one another. Molecular biodynamic alterations lead to differences in cellular membrane receptor expression (up and down regulation of beta-1 and beta-2 adrenergic receptors, for example) and a drive toward myocyte hypertrophy, which leads to anatomic or morphologic remodeling of the heart (cardiac muscle hypertrophy and chamber dilation). It is the anatomic and morphologic dilation that most commonly was associated with heart failure when studied previously. Thus, the myocyte and muscle enlargement noted in patients with dropsy and hypertension reflected this pathophysiologic change. Perhaps, today, the two most important characteristics of heart failure are the molecular biodynamic perturbations that lead to detrimental remodeling of the heart. Figure 32.3 characterizes this remodeling process, demonstrating a dilated and hypertrophied heart compared to a more normal shaped specimen, and also shows the myocyte hypertrophy, which is an important component of remodeling, with interstitial fibrosis that contributes to functional derangements.
A great deal of information has come to light recently regarding the molecular biodynamic abnormalities seen in heart failure. Interestingly, a common theme of many “over” and “under” gene expression knockout animal models has been the induction of cardiac hypertrophy. It is now known that several pathways exist that stimulate a variety of mitochondrial protooncogenes that regulate contractile protein production, causing increases in these important cellular elements such that individual myocytes enlarge in an attempt to augment contractility. Perhaps most important are the neuroadrenergic mediators, such as epinephrine and norepinephrine, angiotensin II, endothelin, and several proinflammatory cytokines, such as interleukin-6. Not to be discounted, however, is the importance of the stretch-mediated hypertrophy pathways. This explains the downward spiraling problems failing hearts demonstrate. As cells enlarge and as the cardiac chamber stretches even further, there is a self-promotion of growth that leads to further deleterious and detrimental cardiac remodeling. The intricacy and overlapping network of signal transduction pathways, which contributes to this vexatious problem, is brilliantly summarized by Katz and shown in Fig. 32.4 (3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree