Fig. 47.1
The difference between nodal (Ca++) and muscle (Na++) action potentials is illustrated. Compared with sodium channels of muscle, nodal calcium channel APs have a less negative resting membrane potential, slower upstroke, absence of phases 1 and 2. These cells inherently undergo spontaneous depolarization (automaticity) as seen by the upstroke of phase 4. Once the membrane potential reaches the threshold level, a new impulse is created
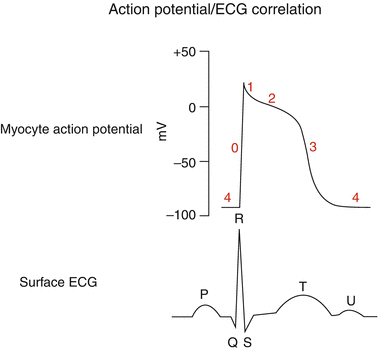
Fig. 47.2
Cardiac muscle action potential with a comparable surface ECG tracing. As illustrated, AP phases are indicated (0–4) and reflect ion exchange concentrations. The ventricular QRS-T of the ECG reflects the intracellular ion flux: QR = phase 0–1, while ST-T = phases 2–3. For this reason, changes in cardiac cellular metabolism, associated with arrhythmias, will often result in ECG changes such as T wave inversion or ST elevations as a marker of intracellular abnormalities
The spontaneous membrane electrical depolarization activity, or automaticity, of nodal cells permits these cells to generate action potentials at a constant rate and act as “pacemakers,” regulating cardiac rhythm. Based on ion channel recovery duration, cells may or may not be able to propagate another AP at any given time interval, a term referred to as “refractoriness.” This property becomes important in the propagation of arrhythmias once they are initiated, and this forms a major focus in pharmacotherapeutic control of arrhythmias. The interdigitating configuration of cardiac cells permits this integrated electrical activity to spread throughout the heart, thus permitting overall muscle contraction. Detailed descriptions of these intra- and intercellular processes can be found elsewhere in numerous reports, and microelectrode voltage-clamp studies and are beyond the scope of this chapter [15]. However, a basic understanding of the normal action potential helps to put some arrhythmias into perspective since any alteration in AP duration, amplitude, or configuration can be the focus for abnormalities of cardiac rhythm (Chap. 46).
47.4 Altered Action Potentials Causing Arrhythmias
As mentioned above, any alterations of normal cardiac cellular action potentials either by disease, ischemia, inherited genetic conditions, cellular chemical composition, drugs, or even simply aging, can be arrhythmogenic. The sinus node becomes less efficient with age, resulting in loss of its “pacemaker” capabilities, causing slower intrinsic heart rates in the elderly but an increase in abnormal escape rhythms due to this lack of rate control. The term “sick sinus syndrome” has been applied to symptomatic arrhythmias associated with sinus bradycardia, sinus arrest, or alternating brady- and tachyarrhythmias. Since sinus nodal control of heart rate begins to decrease after the age of 55 years, the intrinsic ability of heart muscle to generate electrical activity acts against the individual with a reported nearly 25 % lifetime risk of developing atrial fibrillation based on the aging process alone [16]. Any patient with repaired congenital heart, or anyone who has been on cardiopulmonary bypass, can have resultant sinus node damage by virtue of superior vena cava cannulation or sutures. In addition, certain congenital heart defects, such as atrial septal defects, may intrinsically have abnormal sinus node function [17].
Electrolyte abnormalities can affect APs causing atrial and ventricular arrhythmias. Sodium ion overload can occur in the heart tissue resulting in Na-Ca exchange abnormalities, as seen in arrhythmias associated with tissue reperfusion following periods of ischemia. Cardiac muscle acid-base balance can also alter APs. Although a decrease in tissue pH values raises intracellular Na++ and alters contractions by an increase in Ca++ release, a severe decrease to pH 6.7 or less can be associated with Ca++ overload arrhythmias [18]. Abnormalities of the inward potassium channel (IKs), as seen with such inherited genetic conditions such as the long QT syndrome (LQT1), cause AP duration prolongation resulting in membrane instability and a predisposition to often fatal ventricular arrhythmias (polymorphic ventricular tachycardia or torsade de pointe) (Chap. 49). The APs of normal muscle cells, following any metabolic insult such as ischemic injury, can be altered, permitting spontaneous initiation of depolarization. Diseased atrial muscle fibers as well as Purkinje cells can exhibit chronic depolarizations independent of any extracellular activity. Premature atrial contractions (PACs), premature ventricular contractions (PVCs), ectopic atrial tachycardia (EAT/AET), or junctional ectopic tachycardia (JET) can be the result of these adverse membrane changes. Ischemic cells lose potassium causing an increase in extracellular K+ concentrations, which, in turn, alter transmembrane ion ratios, which adversely alter APs [19]. Calcium plays a role in delayed afterdepolarizations contributing to arrhythmias. The normally quiescent membrane ion activity during phase 4 of cardiac muscle APs can become unstable, permitting an increased inward current sufficient enough to trigger a new action potential (early afterdepolarization) and propagate what is referred to as “triggered arrhythmias.” These cellular changes can often be detected by concomitant changes in the S-T, T, and T-U wave patterns seen on the surface ECG (Chap. 48).
Pharmacologic agents, including antiarrhythmic drugs can be proarrhythmic by virtue of adverse action potential effects such as an increasing AP duration or depressing sinus or atrioventricular node activity which can allow for ectopic escape rhythms. Bradycardia contributes to torsade de pointe, while an increase in heart rate can provoke ventricular tachycardia/fibrillation. Drugs or electrolyte abnormalities (hypokalemia) that cause the AP duration to increase have been implicated in fatal arrhythmias. Excellent reviews of pharmacologic agents provoking arrhythmias can be found elsewhere [20, 21] and in Chap. 48. However, a brief listing includes antiarrhythmics (sotalol, ibutilide, and amiodarone), antibiotics (erythromycin and trimethoprin-sulfa), antihistamines (terodiline), and antiseizure agents (thioridazine and tricyclics). In addition, other drugs (psychotropic agents, antifungal agents, chemicals (organophosphates)), as well as liquid protein diets can adversely alter cardiac cell action potentials and provoke arrhythmias.
47.5 Reentrant Arrhythmias
As introduced above, cardiac electrical wave propagation tends to flow relatively smoothly. However, if a wave is blocked in one direction but permitted to reroute and return to the original site of excitation, a revolving pattern of wave propagation ensues: “reentry,” “circus movement.” This concept was first described in the early twentieth century [22]. This circular impulse activity can occur anywhere in the heart in which there is an impediment to electrical propagation (ischemic, anatomical, or mechanical) and is responsible for most of the important clinical tachyarrhythmias. Among these are atrioventricular reentrant tachycardia (AVRT), atrioventricular nodal tachycardia (AVNRT), atrial flutter/fibrillation, and ventricular tachycardia/fibrillation.
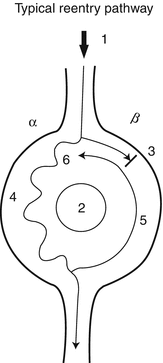
Initiation of a reentrant tachycardia is dependent on several factors: (1) the existence of at least two intersecting pathways or limbs (often referred to as α and β) that create a loop for the wave to travel, (2) the wave propagation must be blocked in one of the limbs of the loop and be allowed to travel down the other limb, and (3) the conduction properties of the tissue creating the loop (speed and refractoriness) must be such that the wave can continue to circle around the loop (Fig. 47.3). Although a patient may be susceptible to having a reentrant tachycardia, intrinsic tissue properties dictate when such an occurrence will happen. For example, a patient with Wolff-Parkinson-White syndrome with accessory atrioventricular pathways that form an anatomical loop with the normal AV node, may exhibit tachyarrhythmias only intermittently. Necessary conditions, often provoked by transient electrical alterations, such as premature atrial or ventricular complexes, need to occur to initiate such an arrhythmia. These reentrant circuits typically do not occur among individuals with completely normal hearts, which is why not everyone complains of abnormal tachycardias. However, even normal hearts can be made abnormal by factors that change myocellular properties.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


