Fig. 56.1
Anatomy and surgical perspective of the tricuspid valve complex. The tricuspid valve consists of three leaflets: anterior (A), posterior (P), and septal (S). There are two main papillary muscles, anterior (a) and posterior (p). The septal papillary muscle(s) is rudimentary and chordae tendinae arise directly from the interventricular septum. Important adjacent structures include the atrioventricular node (AVN), coronary sinus ostium (CS), and the tendon of Todaro, which form the triangle of Koch. Ao aorta, FO foramen ovale, IVC inferior vena cava, SVC superior vena cava, RAA right atrial appendage, RV right ventricle (Reproduced with permission from Rogers and Bolling [6])
Table 56.1
Etiologies of tricuspid regurgitation
Primary (structural) |
Rheumatic |
Myxomatous |
Ebstein’s anomaly |
Endomyocardial fibrosis |
Endocarditis |
Carcinoid disease |
Traumatic (blunt chest injury) |
Iatrogenic (pacemaker/defibrillator leads) |
Right ventricular biopsy |
Drugs (fenfluramine-phentermine or methysergide) |
Radiation |
Secondary (functional) |
Left heart disease (LV dysfunction or aortic/mitral valve disease) |
Any cause of pulmonary hypertension (idiopathic, pulmonary thromboembolism, left to right shunt) |
Any cause of RV dysfunction (myocardial disease, RV ischemia/infarction, chronic volume overload, e.g., from dialysis) |
Atrial fibrillation resulting in RA and tricuspid annular enlargement |
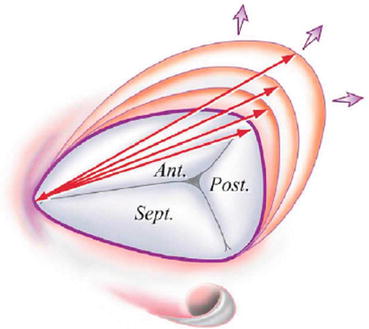
Fig. 56.2
Predominant mechanism of tricuspid annular dilation. Note that in functional tricuspid regurgitation, dilation occurs primarily along the mural portion of the tricuspid annulus, above the right ventricular free wall. Note the shape of the tricuspid annulus is similar to the letter “D,” with the base of the septal leaflet forming the straight portion of the annulus. Arrows designate the intercommissural distance that increases with progressive annular dilation (Reproduced with permission from Dreyfus et al. [11])
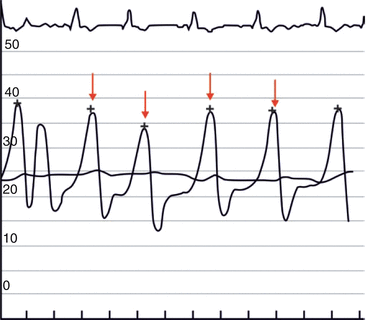
Fig. 56.3
CV Merger waves. Right atrial (RA) pressure waveform shows giant CV merger waves in the presence of severe functional TR with marked elevation in the mean and peak RA pressure (Image courtesy of Jaypee Medical Publishers)
There are often multiple contributors to FTR in a given patient, and the initial management should be targeted at these factors, such as volume overload, left-sided heart failure, and pulmonary hypertension [6].
56.2.1 Natural History of Functional Tricuspid Regurgitation After Mitral Valve Surgery
The primary mechanistic goal of surgery for functional TR should be the application of a rigid annular ring to reduce annular diameter and circumference and thereby leaflet coaptation. Some reports have described that benign neglect of mild-moderate TR at the time of mitral valve surgery may be acceptable since TR is unlikely to progress after MV repair. The fate of TR at the time of mitral valve surgery appears to depend on the etiology of mitral valve disease undergoing correction, and patients with functional MR are at a higher risk for progression of TR after MV surgery (Chap. 55).
For degenerative MR, the fate of TR may be more benign based on some reports. Yilmaz et al. from the Mayo Clinic described 699 patients who underwent mitral valve repair for severe degenerative MR. At the time of surgery, 84 % had TR grade <3+, and 16 % had TR ≥ 3+. These patients were younger (mean, 60 years) and had preserved LVEF (mean, 65 %), and 68 % were NYHA Class ≤2. The mean TR grade at discharge was 1.79 and progressed to 2.11 in those patients with >5-year follow-up. Only one patient required tricuspid reoperation 4.5 years after mitral repair [7]. Matsuyama et al. reported in a study of 174 patients that only 16 % of patients who underwent nonischemic (i.e., degenerative) mitral valve surgery without tricuspid valve surgery developed 3–4+ TR at 8-year follow-up [8]. Other reports do not describe such a benign course for TR after surgery for degenerative MR. Desai et al. studied 1,833 patients with degenerative MR who underwent surgical mitral valve repair. These patients also had a structurally normal tricuspid valve, and 67 with significant TR (almost entirely grade 3–4+) underwent concomitant TV repair. The findings were notable in that all patients undergoing mitral valve repair alone had improvements in TR grade and RV function after surgery; however, these improvements were temporary and by 3 years returned to preoperative levels. In contrast, concomitant TV repair in patients with 3–4+ TR durably eliminated severe TR and improved RV function at up to 3 years. These results support a more aggressive use of surgical TR correction in patients undergoing degenerative MV repair with at least 3–4+ TR at the time of surgery [9].
In contrast, patients with functional MR with TR appear to be at higher risk for progression of FTR over time, and more liberal application of TR correction appears warranted in these patients (Fig. 56.4). Matsunaga et al. described 70 patients undergoing MV repair for functional ischemic mitral regurgitation. Approximately one-third (21/70) of patients had at least moderate TR before surgery. In the postoperative period, the prevalence of at least moderate TR increased over time from 25 % at less than 1 year, 53 % at 1–3 year, and 74 % at greater than 3-year follow-up [10]. For patients with dilated cardiomyopathies (mean LVEF 31 %), DeBonis et al. reported that in 91 patients undergoing MV repair for FMR with varying degrees of preoperative TR, 13 patients (14.2 %) underwent concomitant tricuspid annuloplasty for TR ≥3+, whereas the remaining 78 (with preoperative TR ≤2+) did not. At a mean of 1.8 years of follow-up, 12 % of patients had ≥3+ TR, mostly in those patients who did not undergo tricuspid repair. Based on these findings, the authors argue for more aggressive and effective treatment of functional TR during the initial operation.
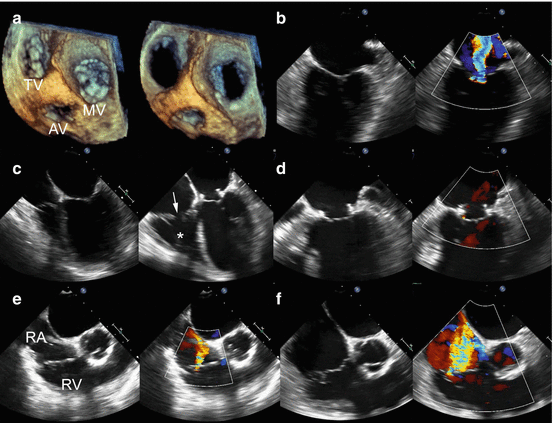

Fig. 56.4
Worsening functional tricuspid regurgitation after surgical mitral valve annuloplasty. (a) 3-dimensional transesophageal echocardiography showing the relationships of the mitral valve (MV), tricuspid valve (TV), and aortic valve (AV) in systole and diastole. (b) Baseline functional mitral regurgitation due to LV enlargement and annular dilation. (c) 14 months after mitral valve annuloplasty showing progressive RV enlargement (asterisk) and tricuspid valve malcoaptation (arrow) due to underlying pulmonary hypertension and chronic systolic heart failure despite MR correction. (d) Continued resolution of MR after mitral annuloplasty with trace MR 14 months after initial operation. (e) Baseline mild TR. RA right atrium, RV right ventricle. (f) 14 months after mitral annuloplasty, severe functional TR is now present, and the patient has symptoms of right heart failure with edema, ascites, and fatigue
It has been proposed by Dreyfus et al. that at the time of MV repair, the presence of tricuspid annular dilation (≥70 mm measured intraoperatively in a flaccid heart by deforming the annulus to its maximal diameter), even in the absence of significant TR, should be an indication for TV annuloplasty. The authors described that TR increased by at least two grades in 45 % of the patients who received isolated MV repair, but TR increased in only 2 % of patients undergoing tricuspid annuloplasty based on their ≥70 mm measurement, supporting the notion that tricuspid dilation is an ongoing, progressive process that often warrants preemptive surgical treatment. Based on their findings, any patient with greater than 2+ TR or a tricuspid annular diameter ≥35–40 mm in any echocardiographic view should be considered for repair of TR during any left-sided valve surgery [11]. Other authors have also reported that preemptive surgical annuloplasty in the presence of tricuspid annular dilation ≥40 mm or ≥21 mm/m2 can stabilize TR grade at a lower level and prevent an increase in RV size over time [12, 13].
56.3 Surgical Approach to Functional Tricuspid Regurgitation
Surgical correction of FTR typically consists of downsizing the tricuspid annular area with ring or suture annuloplasty to move the leaflets closer together. Suture annuloplasty techniques were first described by DeVega and Kay almost 50 years ago [14, 15]. However, despite studies showing more stable and durable outcomes with rigid ring annuloplasties, suture and rigid annuloplasties are still both employed in the surgical treatment of TR [16, 17]. Many of the repair techniques used for the tricuspid annulus were originally described for the mitral valve, which were then translated to the tricuspid valve. Carpentier et al. introduced the concept of rigid ring annuloplasty, which has been applied to the mitral and tricuspid valves [18]. Examples of surgical repair techniques are shown in Fig. 56.5. Newer rigid annuloplasty rings (e.g., MC3 Tricuspid Annuloplasty Ring, Edwards Lifesciences, Irvine California) are shaped to conform to the complex contours of the tricuspid valve annulus.
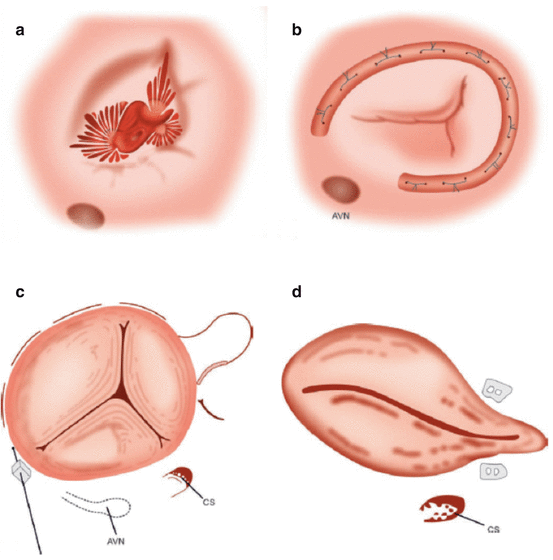

Fig. 56.5
Surgical tricuspid annuloplasty techniques. (a) Dilated tricuspid valve annulus with functional tricuspid regurgitation. (b) Rigid planar annuloplasty ring. Note that the ring is open to prevent damage to the atrioventricular node leading to heart block. (c) DeVega suture repair technique. (d) Kay annuloplasty leading to a bicuspidalized valve (Image courtesy of Jaypee Medical Publishers)
Rigid annuloplasty rings are more durable than suture-based or flexible band annuloplasty for the treatment of FTR. McCarthy et al. reported 790 subjects undergoing TV annuloplasty for FTR using four annular approaches: Carpentier-Edwards semirigid ring, Cosgrove-Edwards flexible band, DeVega procedure, and customized semicircular Peri-Guard annuloplasty. Regurgitation severity increased more rapidly over time with the DeVega and Peri-Guard procedures at up to 8-year follow-up, whereas regurgitation severity was relatively stable across time with the Carpentier-Edwards ring and Cosgrove-Edwards band [16]. Tang et al. have described 702 subjects undergoing TV repair predominantly in the setting of MV surgery, of which 493 had predominantly DeVega repair and 209 had an annuloplasty ring (54 % Carpentier-Edwards, 25 % Duran, and 21 % Cosgrove-Edwards). At up to 21-year follow-up (mean 5.9 years), long-term survival, event-free survival, and freedom from recurrent TR were superior in the ring group. Finally, Navia et al. reported on 2,277 patients who underwent TV procedures during primarily mitral and aortic operations in whom a rigid tricuspid annular ring was used in 26 %, flexible ring in 46 %, DeVega in 5.7 %, Peri-Guard in 8.1 %, Kay procedure in 11 %, and edge-to edge leaflet suture in 3.5 %. By 5 years, TR had increased only slightly to 12 % for isolated rigid prosthesis annuloplasty but was progressively greater for all other annular procedures (flexible prosthesis 16 %, DeVega 24 %, Peri-Guard 44 %, and 19 % for the Kay procedure) [17]. A summary from these comparative reports is shown in Fig. 56.6.
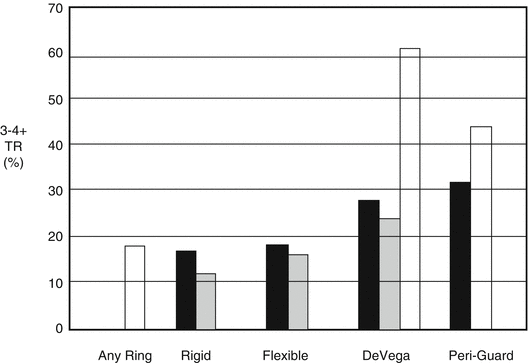

Fig. 56.6
Recurrence of tricuspid regurgitation after ring and non-ring annuloplasty. Reported rates for the recurrence of grade 3 or 4+ TR after initial tricuspid valve annuloplasty by technique. Dark bars, McCarthy et al. 5-year follow-up; gray bars Navia et al. 5-year follow-up; open bars Tang et al. mean 5.9-year follow-up (Reproduced with permission from Rogers and Bolling [36])
Tricuspid valve repair is preferred over replacement. If replacement is required, a bioprosthesis is generally preferred to avoid the bleeding complications associated with anticoagulation for mechanical valves. Despite the common perception, it is not proven that tricuspid valve replacements are at a higher risk for thrombosis than mitral valve replacements. Tricuspid valve repair appears to result in improved midterm survival (up to 10 years after surgery) as compared with TV replacement, although there is no significant difference in valve-related mortality or need for TV reoperation [1]. Although the mechanisms for this difference are not fully understood, it is hypothesized that a rigid object (TV valve) in a deformable low-pressure cavity (right ventricle) may result in RV dysfunction and perioperative low output state. Replacements also complicate the need for right ventricular pacemaker leads. These leads are placed epicardial since this is preferred to leads being placed across the bioprosthesis.
Recently, investigators have examined echocardiographic predictors of adverse outcomes after annuloplasty for FTR. Yiu et al. recently studied 74 patients (age 58 ± 10 years; men 27 %) with significant tricuspid regurgitation who consequently underwent TV annuloplasty during left heart valve surgery [19]. RV mid-cavity diameter, RV longitudinal dimension, and tricuspid valve (TV) tethering area were independently associated with adverse events after adjustment with age and New York Heart Association Class III/IV. Receiver-operator characteristic curve demonstrated that RV mid-cavity diameter (area under curve [AUC] = 0.74, P < 0.01) and TV tethering area (AUC = 0.70, P = 0.04) were most associated with adverse events at 1-year follow-up after TA. Topilsky et al. have shown that the right index of myocardial performance (RIMP) ratio, an echocardiographic global estimate of RV systolic and diastolic function, was a significant predictor of mortality after isolated tricuspid valve replacement [20]. Preoperative assessment of RV function, TV morphology, and the relation of these variables to clinical outcomes remains an understudied area.
Given the data favoring more aggressive treatment of FTR at the time of mitral valve surgery and the superiority of repair over replacement, it is encouraging that several large registry reports have described increased utilization of tricuspid valve repair with annuloplasty and decreased TV replacements. In addition, despite increasing comorbidities and surgical risk, 30-day mortality has decreased perhaps reflecting improved surgical techniques.
Kilic et al. recently reported on 54,375 patients undergoing TV surgery from 2000 to 2010 in the Society of Thoracic Surgeons (STS) National Database [21]. The majority of cases were repairs (89 %) and were performed concomitant with another major procedure (86 %), presumably for predominantly FTR. The most common concomitant procedure was mitral valve surgery alone (47.6 %), followed by CABG with mitral valve (19.6 %) and triple valve surgery with concomitant aortic and mitral valve surgery (12.2 %). The proportion of tricuspid valve repairs increased from 84.6 % in 2000 to 89.8 % in 2010 (P = 0.01). The most common type of repair was tricuspid valve annuloplasty only (75.5 %)—the annuloplasty technique used was not reported. Most of the tricuspid valve replacements were performed using bioprostheses (81.5 %), with the rate increasing from 77.4 % in 2000 to 86.6 % in 2010 (P = 0.001). Despite increased age, comorbidity burden, and more emergency cases over the time period, unadjusted 30-day operative mortality for TV surgery declined from 10.6 % in 2000 to 8.2 % in 2010 (P < 0.001), and this trend persisted after risk adjustment (Fig. 56.7).
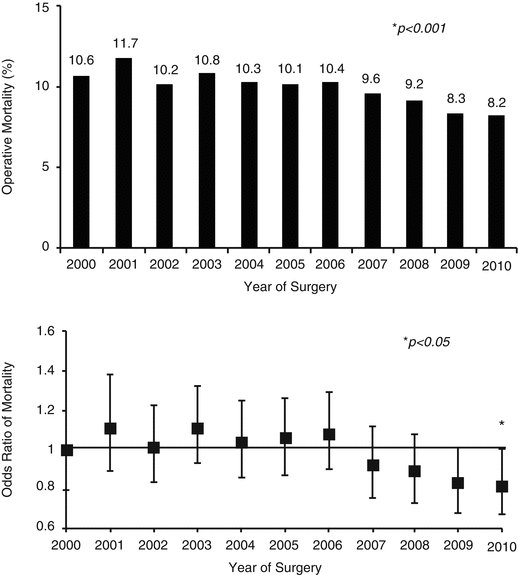

Fig. 56.7
Trends in tricuspid valve surgery in 54,375 patients from STS database 2000–2010. Annual unadjusted (top panel) and risk-adjusted (lower panel) operative mortality rates during the study period (Reproduced with permission from Kilic et al. [21])
Marquis-Gravel et al. reported on a retrospective cohort study of 926 consecutive cases of TV surgery (792 repairs and 134 replacements) performed at a single center from 1977 to 2008 [22]. Operative mortality decreased over time (20 % from 1977 to 1998, 7 % from 1999 to 2008, P < 0.001). Ten-year survival was 49 ± 2 % and 38 ± 5 % in the repair and replacement groups, respectively (P = 0.012). At discharge, severity of TR was ≥3/4 in 13 % of repair and in and 2 % of replacement groups (P = 0.01). Propensity score analysis showed that tricuspid repair was associated with higher rates of TR ≥3/4 at follow-up compared with replacement (hazard ratio 2.15, P = 0.02). Importantly, the use of rigid annuloplasty rings (such as Carpentier-Edwards, Contour, Tri-AD, and MC3) was the predominant repair technique used in the latter part of this series, and the use of a rigid ring resulted in less TR ≥3/4 recurrence at follow-up compared with patients who underwent Bex or DeVega procedures (HR 0.56, P = 0.001). Forty-eight reoperations (7 % of patients at risk) were performed during follow-up (repair group, 6 %; replacement group, 15 %; P = 0.01). At last follow-up, New York Heart Association functional class was improved compared with baseline in both groups (P < 0.001). The authors conclude that tricuspid valve surgery is associated with substantial early and late mortalities but with significant functional improvement [22]. Replacement is more effective in early and late corrections of regurgitation, but it did not translate into better survival outcomes.
Despite these and other data that encourage a proactive and aggressive approach to surgical TR correction, others have reported that a concomitant TV operation at the time of MV surgery is a proxy for more advanced valve disease, and that compared with MV operations alone, combined mitral and tricuspid operations are associated with increased morbidity and mortality even after risk adjustment. LaPar reported on a registry from Virginia over a 6-year period with 5,495 MV operations (approximately half replacements and half repairs), of which 433 had a concomitant TV procedure (25 replacements, 408 repairs). The etiology of mitral valve disease was not described in this registry. Those undergoing the combined valve surgery were more frequently women, had heart failure, required reoperations, and had higher unadjusted operative mortality (6.0 % MV only vs. 10.4 % MV+TV, p = 0.001). Risk adjustment showed that the performance of concomitant TV procedures was an independent predictor of operative death (odds ratio, 1.50; p = 0.03) and major complications (odds ratio, 1.39; p = 0.004) [23] (Chaps. 54 and 55).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


