Fig. 3.1
Mesenteric circulation and its collateral networks between the celiac artery, superior mesenteric artery, and inferior mesenteric artery (By permission of Mayo Foundation for Medical Education and Research. All rights reserved)
Physiologic Response
Approximately 20 % of the cardiac output goes through the mesenteric arteries under normal conditions [10]. Blood flow to the gastrointestinal tract increases even before the ingestion of a meal, remaining elevated at levels approaching 100–150 % of normal (2,000 ml/min) over the next 3–6 h. There is still controversy if the blood flow is redirected selectively to the mesentery. In the 1930s, Herrick used a thermostromuhr to demonstrate that blood flow is increased 5 h after a meal in awaken dogs not only in superior mesenteric artery but also in carotid, coronary, and femoral arteries. Some speculated that these changes could be due to an increase in cardiac output [11]. Most of the early attempts to understand the physiologic response of mesenteric circulation after meal were based in extrapolation from animal data or from limited experiments using angiographic techniques or laparotomy with application of electromagnetic flowmeters [12].
The normal hyperemic postprandial response is mediated by cardiovascular changes that accompany the ingestion and digestion of food. It is well documented that these changes start even before food reaches the stomach. Anticipatory response usually represents a small increase in superior mesenteric artery blood flow. When meal doesn’t reach the stomach, response tends to last only a few minutes. Studies in dogs and primates have shown that cardiac output, heart rate, and aortic pressure are increased in this phase but also that there is little change in mesenteric vascular resistance. After the anticipatory period, increase in cardiac output is not well documented [11].
Mesenteric vasodilatation starts 3–5 min after food enters the intestine, reaching its maximum 30–90 min later and lasting 4–6 h. The latency and duration of these responses depend upon the type and quantity of a meal, with high fat and protein-containing foods producing the most profound and sustained intestinal hyperemia [13]. Moneta and colleagues described variations in duplex scan measurements after ingestion of six different liquid meals by conscious humans: mixed, carbohydrates, fat, protein, mannitol, and water. Superior mesenteric artery blood flow measurements showed significant increases in peak systolic velocity, end-diastolic velocity, mean velocity, and volume flow after all meals, except water. Peak changes after carbohydrate meal tended to occur earlier and to be less intense than after mixed or fat meals. Although increases in duplex parameters in response to protein were less than those of carbohydrate, they appeared to be better sustained. Femoral and celiac arteries showed no significant changes. Minimal change in velocities in the celiac axis (CA) is presumably due to the relative low resistance on the splenic and hepatic circulations at baseline [12].
Postprandial mesenteric hyperemia is confined to organs in which digestion is occurring, but is not shared equally within the same mesenteric arterial territory or within the tissue layers of the intestine. The increased blood flow in the superior mesenteric artery (SMA) territory elicited by food in the intestine is associated with little to no change in blood flow to the stomach, pancreas, and colon. Studies with introduction of food in specific parts of the intestine of dogs showed that even within the intestine, various regions are perfused in different degrees [11]. At the level of the intestinal wall, blood flow distribution favors the mucosa (70 to 80 % of total blood flow), rather than the submucosa and muscularis [14]. Mesenteric postprandial hyperemia is selective in its distribution to regions related to digestive and absorptive processes [11].
Several mechanisms have been proposed to explain postprandial hyperemia. Potential mediators are divided in five categories: direct effect of absorbed nutrients, enteric nervous system, gastrointestinal hormones and peptides, local nonmetabolic vasoactive mediators, and local metabolic vasoactive mediators [15]. Lipid micelles, some amino acids, carbon dioxide, and nitrogen ions are capable of diffuse across intestinal epithelial barrier to directly initiate autoregulation of blood flow in microvessels [16].
Effects of enteric nervous system are unclear. Postprandial hyperemia is not modified by pharmacological or surgical sympathetic blockade. Atropine infusion inhibits food-induced mesenteric vasodilatation, which is compatible with at least a partial influence of a hormonal mechanism, for cholinergic blockade reportedly prevents the release of cholecystokinin (CCK) [11]. Capsaicin-sensitive afferent fibers responsible for releasing CCK, substance P, and vasoactive intestinal polypeptide (VIP) are also potentially involved, since capsaicin and lidocaine can prevent hyperemia associated with micelle absorption. Therefore, a nonadrenergic, noncholinergic mechanism is possible [16].
Earlier studies with systemic infusion of secretin, gastrin, or CCK reported increases in superior mesenteric blood flow. CCK was also associated with increases in small intestinal and pancreatic blood flow [11]. Premen and colleagues questioned the importance of CCK as a physiological intestinal vasodilator, based on the finding that at physiological rates, it didn’t alter intestinal blood flow. These authors performed intra-arterial infusion of secretin, neurotensin, CCK, and a combination of the three hormones in dogs. Results suggested that alone or in combination, none is of quantitative importance in regulating blood flow in postprandial state [17]. VIP, gastric inhibitory peptide, calcitonin gene-related peptide α, glucagon, enkephalins, somatostatin, and peptide YY also don’t appear to have a role at physiological doses. It is acceptable, however, that specific sites in the digestive system may experience sufficient levels of these substances to produce a controlled local effect [16].
Serotonin, histamine, bradykinin, and prostaglandins are produced by small intestine in response to normal or pathological stimuli. Histamine release in the stomach has long been implicated in the control of blood flow. Its vasodilating effects are mainly mediated by H1 receptors. The proper role of local nonmetabolic vasoactive mediators probably depends on the balance of vasoconstrictors and vasodilators [11, 17].
Current knowledge suggests that metabolic products, mainly oxygen uptake and tissue PO2, are the basic mediators of postprandial vascular response. Adenosine occupies fundamental positions in almost every metabolic process, and its levels are also elevated during hyperemia. Nitric oxide (NO) is a potent vasodilator product of endothelium, with important role as a regulator of intestinal motility, fluid balance, and electrolyte absorption. In rodents, NO appears to be essential for permucosal arteriolar dilation [16].
Collateral Pathways
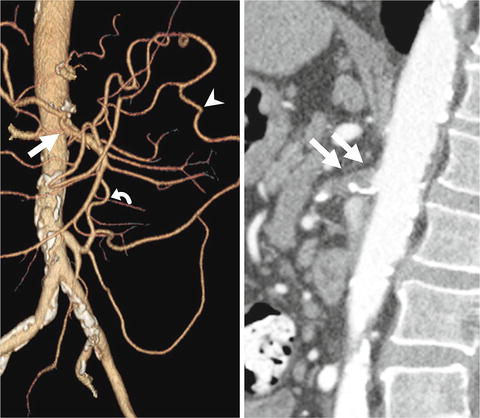
The mesenteric circulation is rich in collateral network between the three main visceral artery territories (CA, SMA, and inferior mesenteric artery) and the internal iliac arteries (Fig. 3.1). Direction of blood flow is contingent on the location of the significant stenosis. The gastroduodenal and pancreaticoduodenal arteries provide collateralization between the CA and SMA. The marginal artery of Drummond and the arc of Riolan (Fig. 3.2) connect the left colic artery (inferior mesenteric artery) to the middle colic artery (SMA). The term meandering or central anastomotic artery describes marked enlargement that occurs in the arc of Riolan in patients with high-grade stenosis or occlusion of the SMA and collateralization via a patent inferior mesenteric artery (IMA) [18]. The internal iliac arteries provide a collateral pathway via the hemorrhoidal branches.