Fig. 10.1
Anatomy of the AV node and bundle of His in relation to the ostium of the coronary sinus, the septal leaflet of the tricuspid valve and the membranous septum. The limits of the triangle of Koch are defined by these structures and by the tendon of Todaro, a subendocardial fibrous cord that constitutes the superior side of the triangle
Bundle of His
It is the continuation of the distal part of the AV node, and is immersed in the fibrous skeleton. It travels forward over the crest of the interventricular septum finishing ahead of the membranous septum, at the vertex of the triangle of Koch (Fig. 10.1).
Left Bundle Branches
They arise from the bundle of His in its forward course and are distributed as a continuous “fan” of cells along the left ventricular septal surface.
Right Bundle Branch
It is the direct continuation of the bundle of His after the origin of the last of the left bundle branches.
10.3 Method of Study
Sinus Node
In order to study the SA node, the right atrium must be opened with scissors, from the entrance of the inferior vena cava towards the atrial appendage (following the dotted line shown in Fig. 10.2a). Next, the atrial wall is opened between both venae cavae, but sparing the anatomical location of the SA node. The crista terminalis is removed as a whole and is cross-sectioned every 3–4 mm to obtain 5 or 6 sections (Fig. 10.3).
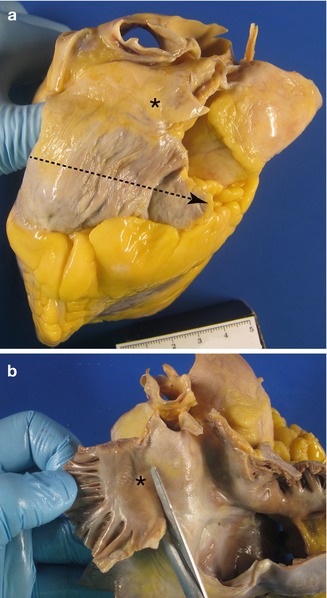
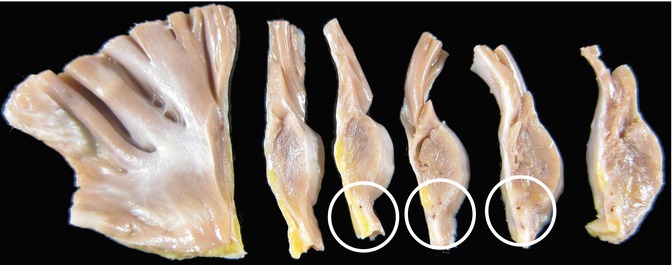

Fig. 10.2
Location of the SA node : (a) as seen from the heart’s surface and (b) from the internal side of the right atrium

Fig. 10.3
Serial sectioning of the crista terminalis. Gross examination allows visualisation of the SA nodal artery and, on some occasions, of the surrounding whitish nodal tissue (circled)
Atrioventricular System
A block of tissue is obtained containing the atrioventricular junction at the level of the insertion of the septal leaflet of the tricuspid valve, from the entrance of the coronary sinus to the septum past the membranous septum (Figs. 10.4 and 10.5a). This block should include 1 cm of the interatrial septum above the tricuspid valve annulus and around 2 cm of the interventricular septum below (Fig. 10.5). This block of tissue is cut every 2–3 mm, obtaining between 6 and 8 sections that are conventionally processed and embedded in paraffin (Figs. 10.5b and 10.6).
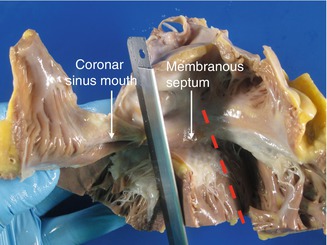
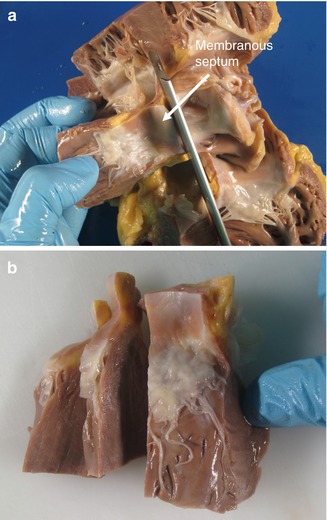
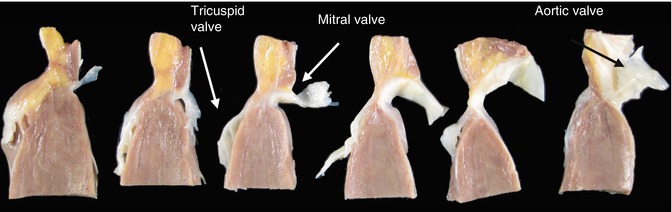

Fig. 10.4
Selection of the block of tissue to study the atrioventricular system. The knife blade points to the excision past the ostium of the coronary sinus. The discontinuous line indicates the direction of the excision, parallel to the previous one, in the anterior part of the septum

Fig. 10.5
(a) Superior excision of the block, 1 cm above the tricuspid valve annulus. (b) Further sectioning of the block in sections 2–3 mm in width

Fig. 10.6
Image showing the different sections obtained after serial sectioning of the block
Each of the sections obtained is automatically processed according to standard methods and the slides are stained with haematoxylin-eosin and Masson’s trichrome. This is the simplest method that allows visualisation of all the elements of the CCS although it has many limitations, especially to identify anomalous pathways (see below). The optimal method (only possible in research laboratories) is the manual embedding of the tissue in paraffin and the serial sectioning of the entire block, obtaining more than 1,000 slides for every conduction system. An intermediate approach consists of obtaining serial sections of the blocks that have been automatically processed, and staining every 15–20 slides.
10.4 Histology of the Cardiac Conduction System
10.4.1 The Normal Cardiac Conduction System
Sinus Node
The SA node is composed of fusiform cells, with a diameter smaller than those of the atrial cells, and the interstitium contains large amounts of elastic tissue and collagen, which increase with age. The nodal tissue is typically crossed by the SA nodal artery, which can appear as a single artery or can branch into two or more arteries, and frequently contains many nervous tracts and neurovegetative ganglions, especially in the periphery (Fig. 10.7a).
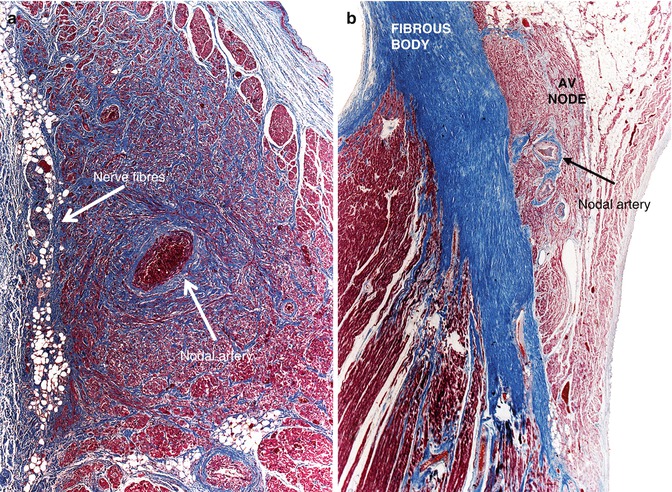

Fig. 10.7
(a) Normal sinus node. (b) Position of the AV node above the central fibrous body
Atrioventricular (AV) Node
It is an ovoid structure with a maximum length of 5–8 mm. It can be divided into a poorly-defined portion, composed of fascicles arranged parallel to one another, and a portion that corresponds to the proper compact node that sits above the central fibrous body (Fig. 10.7b).
Bundle of His
It gradually arises from the AV node, which begins penetrating the central fibrous body up to a point where all the conduction cells are completely included in it (this region is termed the penetrating bundle) (Fig. 10.8a). This part is very short and from the His bundle exit conduction fibres forming a cascade down the left ventricular septal surface (the left branches). This is the so-called branching bundle, situated beneath the commissure of the right and non-coronary aortic sigmoid cusps, below the membranous septum, adopting a triangular shape (Fig. 10.9b).
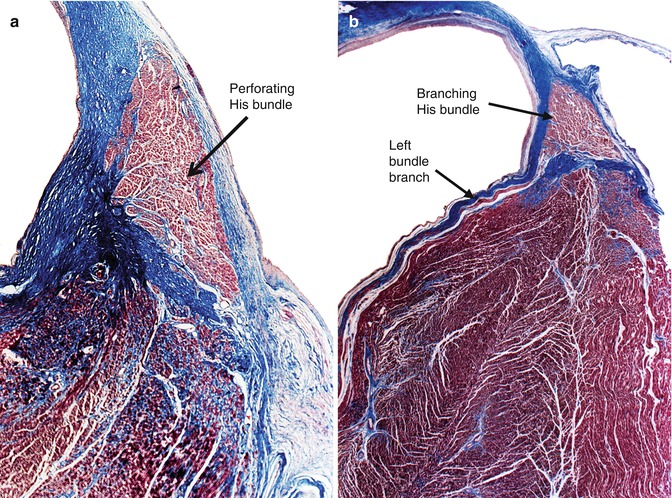
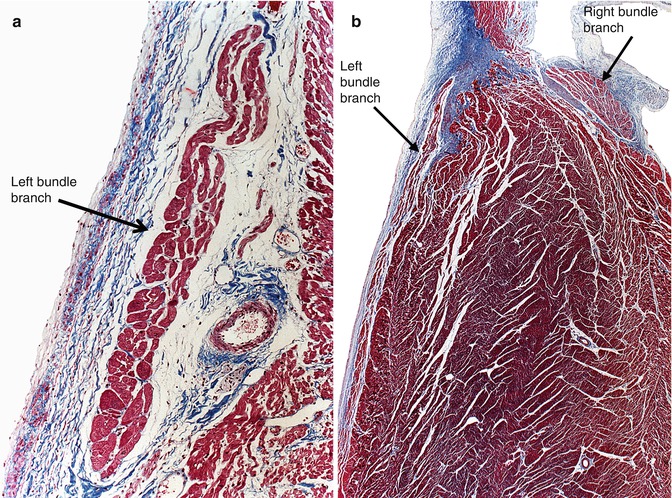

Fig. 10.8
(a) Penetrating portion of the bundle of His within the central fibrous body. (b) Branching portion of the bundle of His situated at the crest of the interventricular septum, and one of the left branches travelling down the left side of the septum

Fig. 10.9
(a) Detail of the left bundle branch under the endocardium. (b) Image showing the emergence of the last of the left bundle branches and the initial portion of the right bundle branch (Masson’s trichrome)
Left Bundle Branches
Left bundle branches are multiple and originate from the bundle of His as a continuous “fan” of cells. The left bundle branches primarily travel subendocardially along the left ventricular septum, and an interstitium of lax connective tissue separates the left bundle branches from the contractile myocardium. The cells vary in shape in comparison with the ventricular cells and can be compact or vacuolated. These are the so-called Purkinje cells (Figs. 10.8b and 10.9).
Bifurcation
It is the end of the His bundle, from where the last of the left bundle branches and the right bundle branch emerge.
Right Bundle Branch
It is the continuation of the bundle of His once all the left branches have arisen (Fig. 10.9b). Moving down the right side of the interventricular septum, it travels intramyocardially near its origin, whereas in the medium and lower thirds of the septum it becomes a subendocardial structure. Histologically, it is similar to the ventricular myocardium and it commonly contains one blood vessel and adipose tissue.
10.4.2 Normal Variations
The study of hundreds of hearts from persons who died due to non-cardiac causes has allowed the recognition of several patterns of morphology, which, despite the fact that they do not follow the previously described anatomy, have no functional consequences in the majority of cases. The most common variations are as follows:
10.4.2.1 Persistent Foetal Dispersion of the AV Node and His Bundle Fragmentation
In many hearts, the conduction system does not form a compact structure but rather forms islands or tongues of conduction tissue, adopting an “archipelago” type morphology. This variation for the AV node is known as persistent foetal dispersion since this is the typical morphology of the AV node during normal development (Fig. 10.10a). The septation of the His bundle is characterised by fragmentation of the main bundle forming loops (Fig. 10.10b). For some time, these findings in adults were considered the cause of death in cases of sudden cardiac death where no other structural alterations were present; however, it was later established that these were normal variations of the CCS.
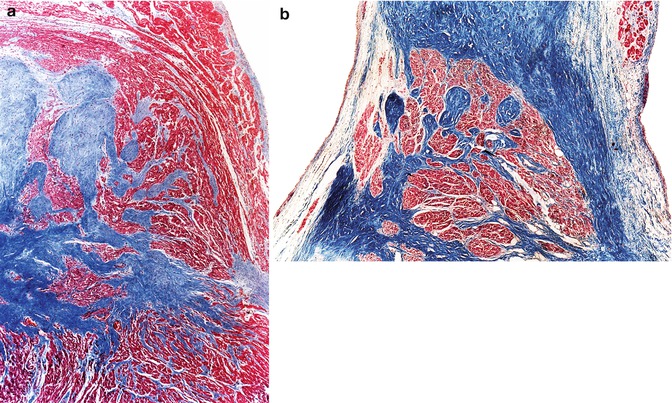

Fig. 10.10
(a) A 2-year-old boy who died suddenly from an endodermal sinus tumour in the mediastinum. The AV node penetrates the central fibrous body as tongues of conduction tissue. (b) A 48-year-old male who died from severe brain injury after falling from a height of 3 m. The His bundle presents a polylobulated morphology (Masson’s trichrome) (Image reproduced with permission of Suárez-Mier and Gamallo 1998)
10.4.2.2 Variations in the Position of the His Bundle
It is relatively common for the bundle of His, especially its branching portion, not to be located as shown in Fig. 10.8, astride the interventricular septum, but rather in different positions, as illustrated in Fig. 10.11. Positions shown in C and D are very uncommon and their functional consequences are uncertain.
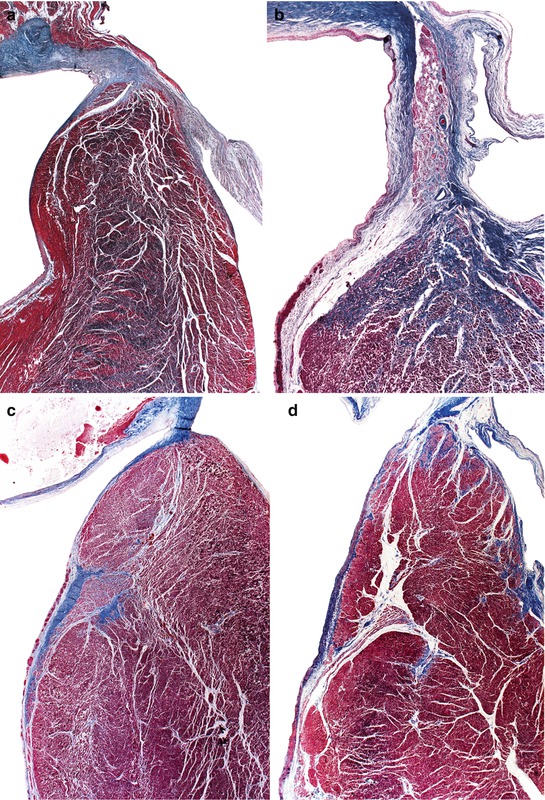

Fig. 10.11
(a) A 21-year-old male who died suddenly with no known medical history. Heart weight, 403 g. Bundle of His situated above the septum, displaced to the left. (b) An 81-year-old woman with no cardiac history. The His bundle is situated in the membranous septum. (c) A 32-year-old male. Work-related accident involving crushing. The bundle of His is in a much-lowered position, at the left side of the septum. (d) A 55-year-old male. His heart showed a healed anterolateral infarct with 90 % luminal stenosis in the left coronary trunk. The bundle of His is much lowered down and intramural (Masson’s trichrome)
10.4.3 Age-Related Changes
10.4.3.1 Adipose Tissue Infiltration
Adipose tissue infiltration is a degenerative alteration of the CCS that appears around the age of 50 years and involves all the structures of the conduction system, with the nodes being the most affected structure followed by the His bundle bifurcation (Fig. 10.12).
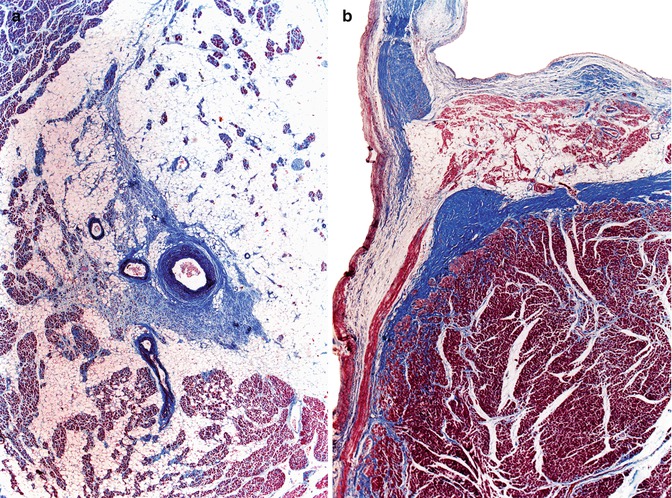

Fig. 10.12
(a) Marked adipose tissue infiltration in the sinus node, in a 68-year-old male with a history of chronic bronchitis. (b) Adipose tissue infiltration in the bifurcation of the His bundle in a 59-year-old male who died suddenly from ischaemic heart disease (Masson’s trichrome)
10.4.3.2 Fibrosis
Fibrosis of the CCS is an age-related alteration, although it has also been associated with sick sinus syndrome, congenital block, collagen diseases and chronic atrioventricular block (see below) (Fig. 10.13).
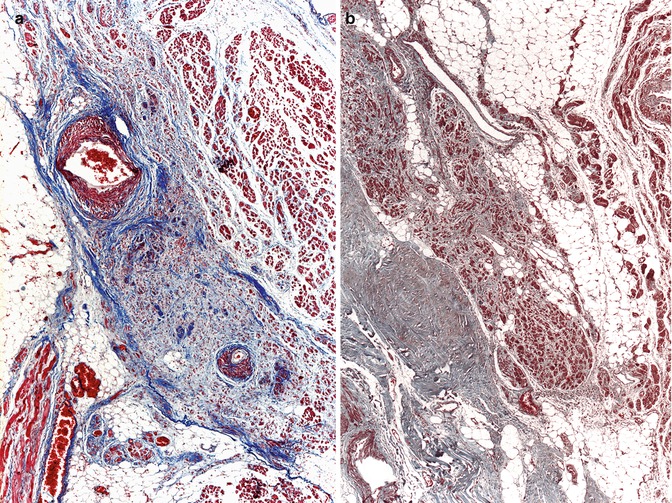

Fig. 10.13
(a) Fibrosis of the sinus node in a 90-year-old woman. (b) AV node with fibrosis and mild adipose tissue infiltration in a 52-year-old male carrying a pacemaker (Masson’s trichrome)
10.4.4 Haemorrhage Secondary to Resuscitation Manoeuvres
In older literature dealing with sudden cardiac death in infants, haemorrhage in the CCS was thought to have a hypoxic origin, associated with respiratory problems, and was considered a possible cause of death. However, haemorrhage in the CCS is a lesion commonly seen in individuals who suffered sudden death and received cardiopulmonary resuscitation, as a result of the compression of the heart between the sternum and the spine during cardiac massage (Fig. 10.14).
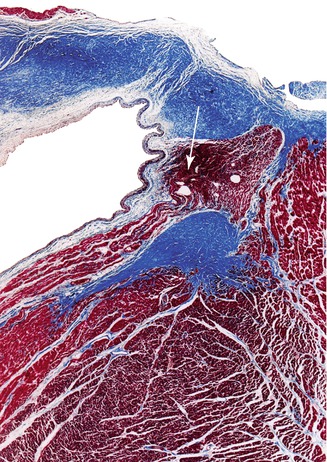

Fig. 10.14
Haemorrhage in the bundle of His (arrow) (Masson’s trichrome)
10.5 Pathology of the Cardiac Conduction System
Clinical and histologic studies of the human conduction system do not always correlate, and alterations from what it is considered the normal morphology of the CCS are in many cases not associated with functional defects; moreover, alterations in the cardiac rhythm that have been clinically demonstrated even by electrophysiological studies, may not correspond to any morphological lesions. This is especially true for the pre-excitation syndromes, where identification of the anomalous conduction tracts between the atria and ventricles is extremely difficult. In this section we will review some pathologic conditions localised to the conduction system and others that, despite being non-specific, can manifest as conduction blocks and/or sudden death.
10.5.1 Sick Sinus Syndrome
The sinus node is the area of the heart that under normal conditions controls the cardiac rhythm. Sick sinus syndrome is the name given to a disorder where the sinus node does not function properly. It is generally caused by the aging of the node and/or surrounding atrial cells. Histologically, there is a replacement of the myocardial cells by fibrous tissue (Fig. 10.13a). Dysfunction of the sinus node can manifest as prolonged sinus pauses, symptomatic sinus bradycardia, sinus arrests, sinoatrial block, asystole following cardioversion or the presence of chronotropic incompetence. Sick sinus syndrome can be clinically associated with alterations in the atrioventricular conduction system or with paroxysmal supraventricular tachycardia, known as bradycardia-tachycardia syndrome.
Mortality in patients affected by sick sinus syndrome is significant and is not always due to cardiac causes. In the MOST study, by Flaker et al. 2003, from a total of 2,010 patients, 404 (20 %) died after a medium follow-up period of 33 months. The cause of death was cardiac in 35 % of the cases, non-cardiac in 49 %, and unknown in the remaining 16 %. The main predictive factors of death were old age, male gender, weight, and presence of a previous AMI or dilated cardiomyopathy. Sick sinus syndrome has been associated with sudden death only in anecdotal cases, although the difficulty in demonstrating this correlation makes for the possibility that the association is underestimated.
Treatment involves permanent pacemaker implantation once the manifestations of the disease are associated with symptomatology.
10.5.2 Atrioventricular Block
Atrioventricular block is defined as the delay or interruption of the electric impulse between the atria and ventricles of the heart, caused by an anatomical or functional alteration in the CCS. AV block can be classified as first-degree block if the electrical impulse moves more slowly than normal through the AV node but all the auricular impulses reach the ventricles; second-degree block, when some of the electrical signals do not reach the ventricles; and third-degree or complete AV block, when no atrial stimulus reach the ventricle. In general, the severity of the symptoms increases with the degree of the block. Presyncopal episodes, syncope or malignant ventricular arrhythmias secondary to the low heart rate can occur in complete heart block associated with slow escape rhythms or asystole. The association of AV block with sudden cardiac death is better documented than in the sick sinus syndrome, although its real relevance is unknown.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


