Congenital and developmental disorders
Transposition of great arteries, hypoplasia of the aorta, double aortic arch, aortic arch interruption (often associated with aortic coartaction) aortic valve malformations (supravalvular stenosis, bicuspid aortic valve and aortic coartaction)
Degenerative and acquired disorders
Atherosclerosis, aortic aneurysms, aortic dissection, intramural haematoma, penetrating atherosclerotic ulcer, aortitis (Takayasu, giant cells, syphilitic, tuberculous, collagenopathies)
Disorders associated with heredofamilial syndromes and connectivopathies
Marfan syndrome, Beals-Hecht syndrome, type IV Ehlers-Danlos syndrome, Loeys-Dietz syndrome
Traumatic pathologies
Caused by thoracic (closed or penetrating), abdominal and pelvic trauma. Iatrogenic lesions due to invasive (aortocoronary bypass) and non-invasive (catheterism) surgical treatments can also be included in this category
In this chapter we will focus on the five main aortic pathologies with greater frequency and incidence as a cause of death, many times with a sudden and unexpected onset: atherosclerosis, aneurysms, acute aortic syndrome, coartaction and aortitis.
3.2 Atherosclerosis
Atherosclerosis is the most prevalent aortic disease and is morphologically similar to coronary artery disease (see Chap. 5). The main characteristics of atherosclerosis are summarised in Table 3.2.
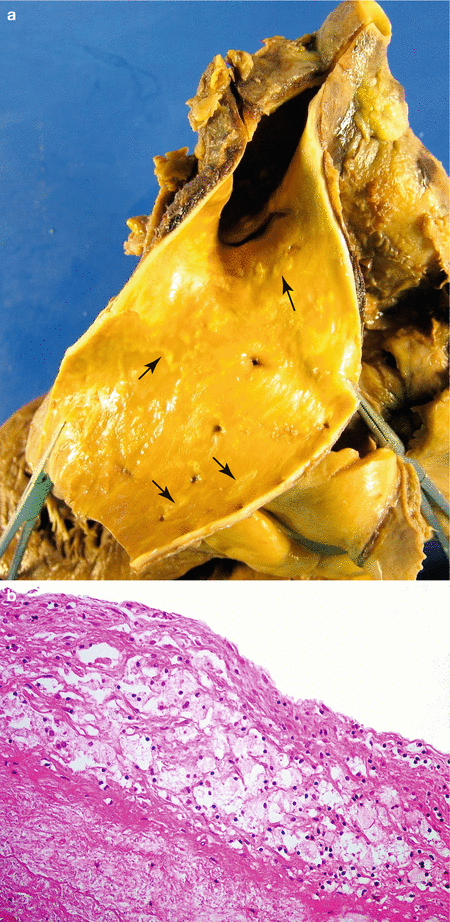
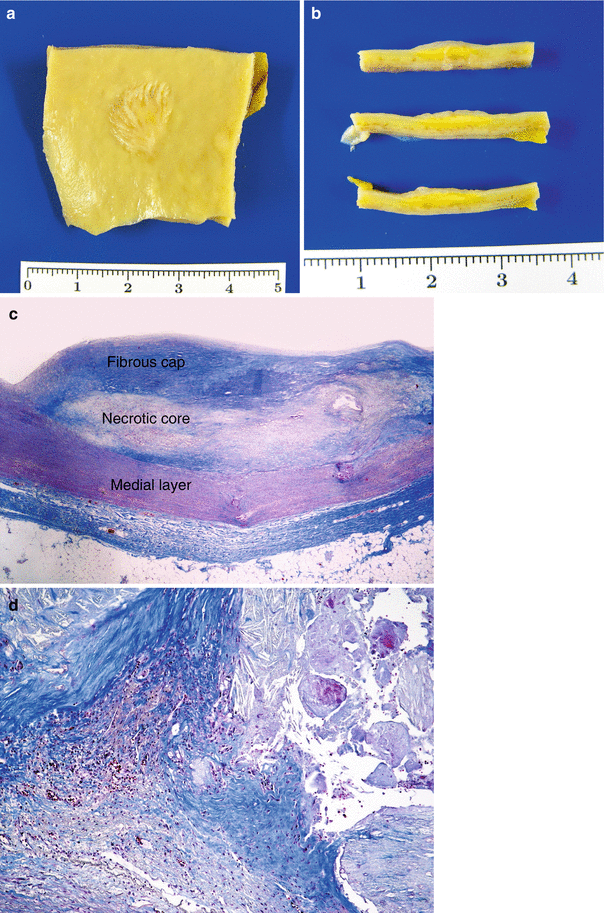
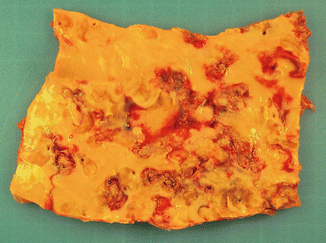
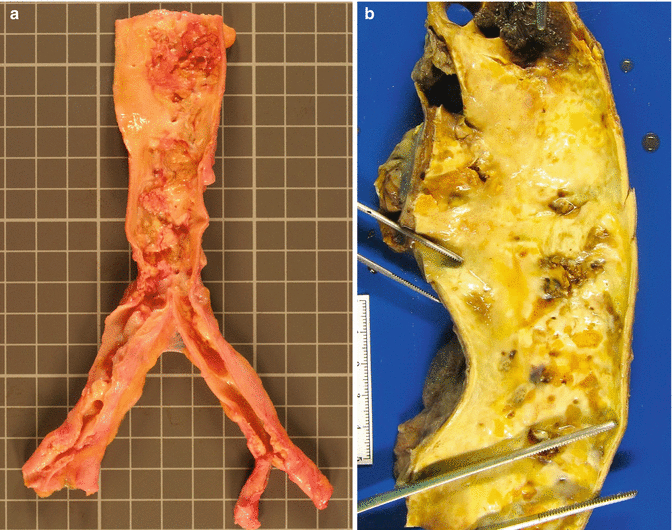
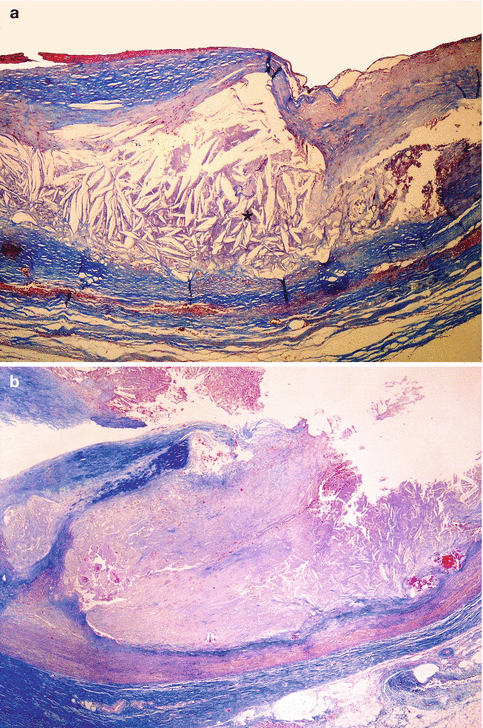
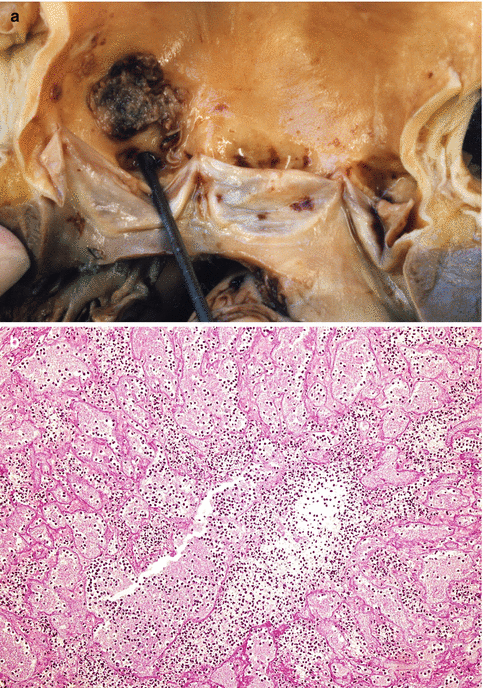
Table 3.2
Pathology of aortic atherosclerosis
Fatty streaks (intimal xanthomas): (Fig. 3.1) |
Yellowish plaque on the intimal surface |
Microscopically: lipid deposits in macrophages and smooth muscle cells (foam cells) and lipid accumulation in the extracellular matrix |
Fibroatheromatous plaques: (Fig. 3.2) |
Bulging lesions that may calcify and ulcerate |
Fibrous cap formed by smooth muscle cells and dense extracellular matrix |
Macrophages, smooth muscle cells and T lymphocytes present under the cap and towards the sides (in the plaque shoulders) |
Internally, necrotic core containing disorganized lipid areas (cholesterol and cholesterol esters), cholesterol crystals, fragments of dead cells, foam cells, fibrin, thrombosis and other plasma proteins |
Neovascularization in the periphery of the lesion |
Fibrous plaques are formed by smooth muscle cells and fibrous tissue |
Ulceration, thrombosis and calcification |
Can occlude the origin of the intercostal and mesenteric arteries. |

Fig. 3.1
Fatty streaks. (a) Fatty streaks in descending aorta (arrows). (b) Microscopic image where foam cells can be observed in the intima

Fig. 3.2
Fibroatheromatous plaque. (a) Macroscopic image of an aortic plaque. (b) Serial sectioning of the lesion where the yellowish lipid content can be appreciated at depth. (c) Microphotography of a plaque at low magnification. (d) Detail of a plaque border at medium magnification where neovascularization can be observed (Masson’s trichrome)

Fig. 3.3
Ulcerated atheromatosis in the thoracic aorta of a 75-year-old male who died suddenly from an acute coronary syndrome

Fig. 3.4
A 61-year-old male with a previous history of hypercholesterolaemia and intermittent claudication in the lower extremities who died suddenly in the street from an acute coronary syndrome. (a) Complicated and calcified aortic atheromatosis involving the abdominal aorta and both iliac arteries. (b) Thoracic aorta with calcified and ulcerated plaques from a 94-year-old woman with generalised atherosclerosis

Fig. 3.5
Complicated plaques. (a) Histological image of an atheroma plaque with calcification and cholesterol crystals (*). (b) Microphotography of an ulcerated plaque (Masson’s trichrome)

Fig. 3.6
A 56-year-old woman with pressure ulcers and gangrene and multiple ulcers caused by pressure and septic liver. (a) Septic mural thrombus over an atheroma plaque in the ascending aorta. (b) Microscopic image of the thrombus with numerous neutrophils
3.3 Aortic Aneurysms
An aneurysm is defined as an abnormal dilatation in a blood vessel or the cardiac wall. When an aneurysm is surrounded by arterial wall components or by an attenuated cardiac wall, the term true aneurysm is used. Conversely, those gaps in the vascular wall leading to an extravascular haematoma that freely communicates with the intravascular space (“pulsatile haematoma”) are called false aneurysms or pseudoaneurysms.
Aortic aneurysms can be classified according to their aetiology, morphology and location (Table 3.3).
Table 3.3
Classification of aortic aneurysms
By aetiology |
Congenital |
Inflammatory or infectious |
Degenerative (the most frequent) |
Associated with hereditary syndromes: Marfan, Beals-Hecht, Ehlers-Danlos or Loeys-Dietz syndromes |
Traumatic |
Iatrogenic |
By morphology: |
Saccular: only one part of the arterial wall is compromised |
Fusiform: the entire circumference of the aortic wall is dilated |
By location : |
Abdominal |
Thoracic |
Annuloaortic ectasia |
Infectious aneurysms can originate from supurative infections that invade, by proximity, the aortic wall; by septic thrombi embolization (mycosis and bacterial infections); or, by direct infection of the aortic wall caused by circulating microorganisms (e.g., syphilis). A non-infectious vasculitis, by bacterial or fungal infections or by formation of immunocomplexes or cross reactivity has also been described.
Traumatic aneurysms can be produced by direct action in cases of penetrating traumatisms affecting the arterial wall or by the action of bone fragments in some closed traumas. They can also be caused by indirect acceleration-deceleration mechanisms in cases of non-penetrating traumatisms. Aortic lesions caused by closed trauma have been observed in the thoracic aorta, mainly at the level of the aortic isthmus (between the origin of the left subclavian artery and the ligamentum arteriosum), since it is the anchor point between the fixed aortic arch and the relatively more mobile thoracic aorta, and therefore sensitive to traction forces exerted by its mobile parts during trauma. The aorta can also become damaged by direct compression against the spinal cord or even by an excessive increase in the intraluminal aortic pressure. These traumatic lesions tend to provoke partial or complete ruptures of the aortic wall (Fig. 3.7) and false aneurysms that can remain contained for several years, leading to ruptures or dissections.
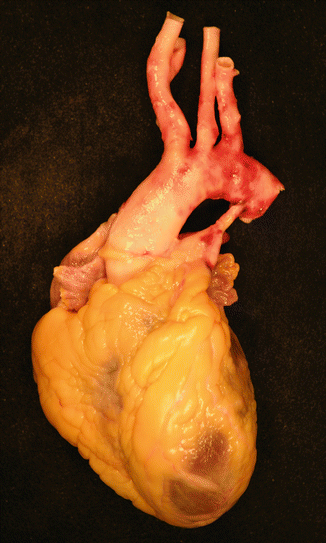

Fig. 3.7
Complete traumatic rupture of the thoracic aorta just below the origin of the subclavian artery, in a 39-year-old woman, caused by a motorbike traffic accident
3.3.1 Abdominal Aortic Aneurysms (AAA)
AAA constitutes the majority of aortic aneurysms and occurs in 2–5 % of the population over the age of 60, and in 10 % of the population over the age of 80. The majority of patients (75 %) are totally asymptomatic or present nonspecific symptoms such as epigastric or lumbar pain, gastrointestinal symptoms or a pulsatile abdominal mass, although the first clinical manifestation can be haemorrhagic shock and sudden death.
The majority of these aneurysms have an atherosclerotic origin and the main risk factors are: smoking, old age, hypertension, and male gender (4–8:1). There are also familial cases; however, AAA due to hereditary diseases such as Marfan’s syndrome are very infrequent.
The major complication of AAA is rupture and free bleeding into the peritoneal cavity (aneurysms with anterior rupture) or into the retroperitoneal circundant tissues (Fig. 3.8). The clinical presentation with hypovolemic shock is usually faster in aneurysms with anterior intraperitoneal rupture. The risk of rupture is proportional to the size of the AAA. Thus, in AAA with a diameter of less than 4 cm, the risk of rupture is very low; between 4 and 4.9 cm, the risk is around 1 % per year; between 5 and 5.9 cm around 11 % annual; and with AAA larger than 6 cm, the risk is about 25 % per year. The progression of the diameter of the aneurysm depends principally on the blood pressure; therefore uncontrolled hypertension is associated with a faster progression in the size of the aneurysm.
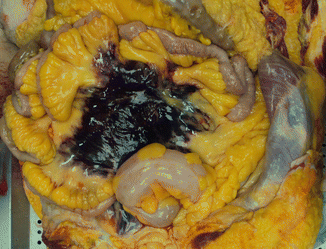

Fig. 3.8
Retroperitoneal haematoma secondary to the rupture of an abdominal aortic aneurysm. An 80-year-old woman who was dead on arrival to the hospital
Other less frequent complications include thromboembolic events due to embolia in ulcerated atheromas or mural thrombi, compression of adjacent structures (ureters, vertebrae), rupture with fistulization into the neighbouring organs, such as the inferior vena cava or the digestive tract (mainly the duodenum), or compression in arterial branches with ischaemic tissular lesion, particularly in the iliac (claudication), renal, mesenteric or vertebral (bone marrow ischaemia) arteries.
The atherosclerotic form gives rise to saccular or fusiform aneurysms, occasionally reaching up to 15 cm of diameter and with variable length. The arterial wall is thinned, with a destructured medial layer, making it more fragile and susceptible to spontaneous ruptures (Figs. 3.9 and 3.10). The aneurysm and adjacent aorta tend to have atheromatous ulcers covered by granular mural thrombi. The lumen in an aortic aneurysm is frequently occluded by a white-yellowish fibrin laminar thrombus. The destructured medial layer, together with the mural thrombi, can obstruct the lumen of the arterial branches, which might explain the acute and chronic distal ischaemic phenomena. In practice, the majority of ruptured atherosclerotic AAA are larger than 8 cm in diameter, are located at an infrarenal level, and extend toward the iliac bifurcation (Fig. 3.11). Occasionally, they can be associated with aneurysms in the primitive iliac arteries (Fig. 3.12).
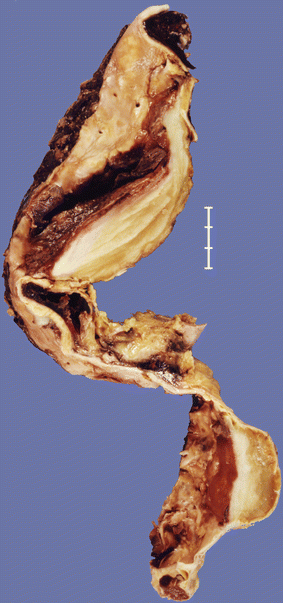
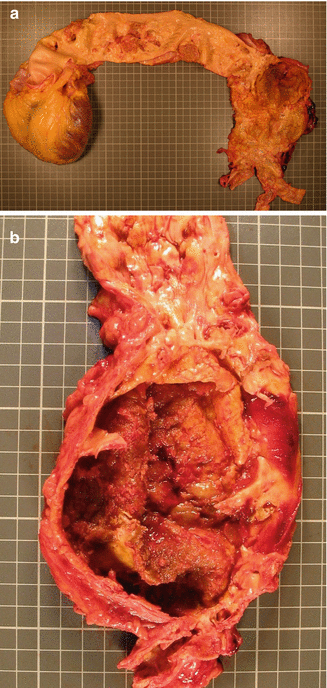
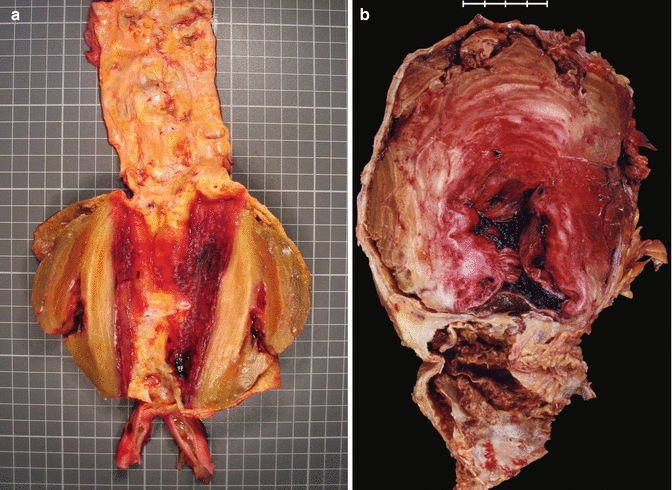
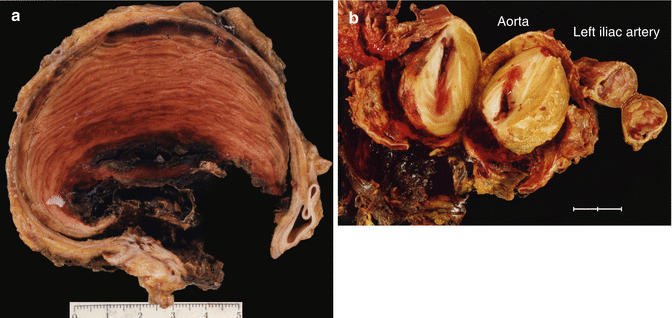

Fig. 3.9
An 81-year-old male who presented with two thrombosed atherosclerotic aneurysms, in thoracic and abdominal aorta respectively

Fig. 3.10
(a) A tear in an atherosclerotic abdominal aortic aneurysm (AAA) in a 78-year-old male with a previous history of hypertension who died suddenly. (b) Detail of the atherosclerotic and fusiform abdominal aneurysm with an infrarenal location

Fig. 3.11
(a) Longitudinal section of an abdominal aortic aneurysm showing laminar thrombosis. (b) Large thrombosed aneurysm in the abdominal aorta located below the origin of the renal arteries. Woman of 84 years with ischaemic cardiomyopathy

Fig. 3.12
(a) Subocclusive laminar thrombosis in a transversal section of a distal abdominal aortic aneurysm in a 76-year-old male. (b) Thrombosis in a ruptured abdominal aneurysm with circundant haemorrhage. The left iliac artery also presents a thrombosed aneurysm
3.3.2 Thoracic Aortic Aneurysms (TAA)
Thoracic aortic aneurysms comprise seven main categories (Table 3.4):
Table 3.4
Thoracic aortic aneurysms
Type of aneurysm | Location |
|---|---|
Atherosclerotic/degenerative aneurysms | Descending aorta and aortic arch |
Dissecting aneurysms | Ascending or descending aorta (described later) |
Aneurysms associated with cystic medial degeneration | In aortic root (annuloaortic ectasia) and ascending aorta |
Aneurysms caused by congenital weakness of an aortic sinus | Sinus of Valsalva |
Pseudoaneurysms (generally secondary to trauma) | Close to the ligamentum arteriosus |
Inflammatory aneurysms (secondary to Takayasu aortitis in the young and to giant cell aortitis in the elderly) | Ascending aorta (described later) |
Infectious aneurysms (mycotic) | Descending aorta |
Aneurysms which are located in the proximal portion of the aorta can affect the aortic root (formed by the fibrous annulus, the Valsalva sinuses, the leaflets and the sinotubular junction) and/or the ascending aorta (from the sinotubular junction to the right brachiocephalic trunk).
3.3.2.1 Aneurysm of Sinus of Valsalva
Aneurysms in the sinuses of Valsalva are the least frequent and occur due to a weakness in the aortic wall that forms part of the sinus. This causes dilatation and the formation of a blind pocket in one of the aortic sinuses (usually the right sinus and less frequently the posterior one). The majority of sinus of Valsalva aneurysms are congenital lesions tightly associated with ventricular septal defects, although they can also be associated with aortic coarctation and with the presence of a bicuspid aortic valve. The age of onset ranges from 10 to 70 years with an average of 35 years.
Its clinical presentation is related to rupture that occurs between the ages of 20–40 years, although some cases of rupture have been described in childhood and also in the elderly. The physiological consequences of this rupture depend on the speed at which it is produced, the size of the ruptured orifice and the cavity it ruptures into. Aneurysms occurring in the right sinus generally rupture into the RV, those of the posterior sinus rupture into the right atrium (Fig. 3.13), and left sinus aneurysms rupture into the pulmonary artery, left ventricle, myocardium or epicardium. Some aneurysms of the posterior Valsalva sinus can rupture into the pericardial cavity causing sudden death by cardiac tamponade.
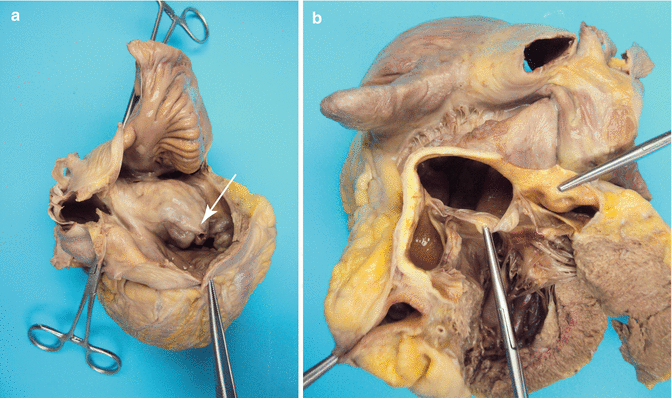

Fig. 3.13
(a) Giant ruptured aneurysm in the posterior Valsalva sinus bulging into the right atrium (the arrow points to the rupture). (b) Aneurysm viewed from the aorta. A 47-year-old woman who died suddenly during sexual intercourse
3.3.2.2 Annuloaortic Ectasia and Ascending Aorta Aneurysms
Annuloaortic ectasia is a symmetrical dilatation of the ascending aorta and the aortic root that can lead to a traction and consequent lack of contact of the valve leaflets, giving rise to aortic valve insufficiency (Fig. 3.14).
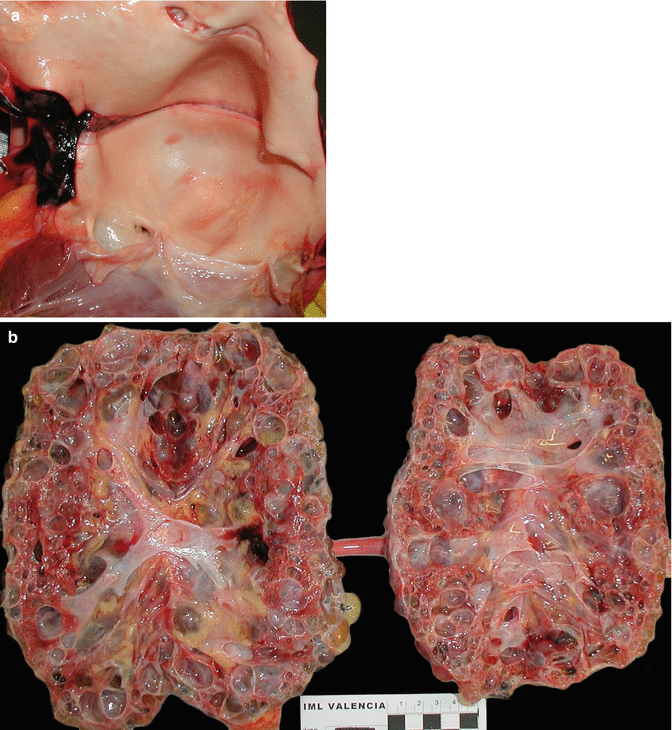

Fig. 3.14
A 39-year-old woman with a personal and family history of renal polycystosis without hypertension who died suddenly. (a) A large dilatation of the aortic root is observed (annuloaortic ectasia) associated with bicuspid aortic valve and transversal intimal dissection, origin of a parietal dissection (arrows). (b) Kidneys with a notable increase in size and weight (R: 1,140 g; L: 865 g) in which a replacement of the renal parenchyma with multiple cysts of various sizes can be observed (Molina et al. (2007). Reproduced with permission from the Editor of EJ Autopsy)
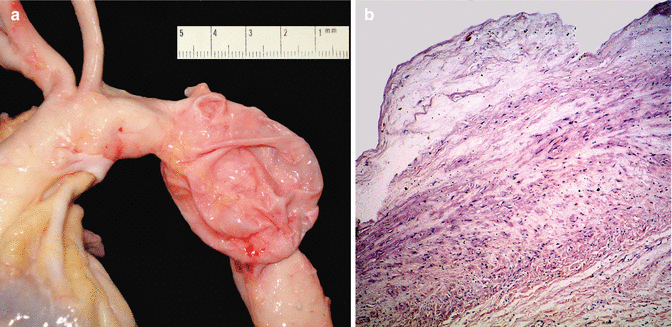
Aneurysms of the ascending aorta can present as an isolated pathology, be associated with a bicuspid aortic valve or be part of several syndromes such as Marfan syndrome, type IV Ehlers-Danlos or Loeys-Dietz syndromes. Histologically, a cystic medial degeneration (CMD) can be observed, which leads to weakening of the aortic wall and confers high risk of aortic dissection (Fig. 3.15). Exceptionally, aneurysms secondary to this type of wall lesion can affect the descending aorta (Fig. 3.16).
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





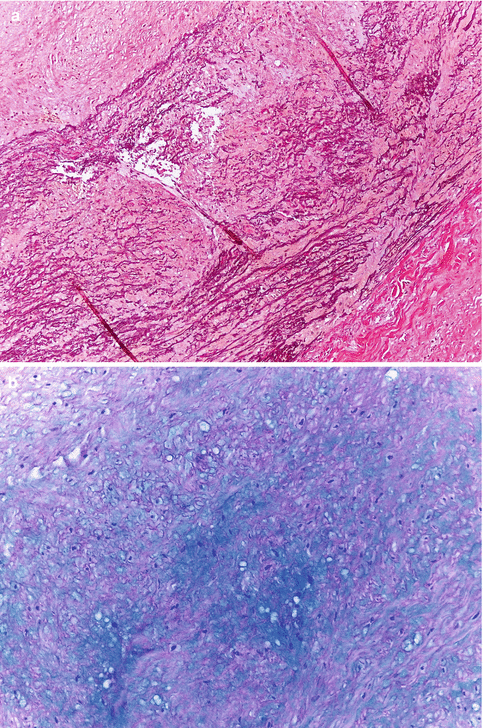
Fig. 3.15
Cystic medial degeneration. (a) Interruption of elastic fibres with formation of empty spaces (Weigert’s stain). (b) Muccopolysaccharide deposit in the areas of elastic fibre loss (Alcian blue stain) (Courtesy of Maria Soledad Sánchez de León. INTCF. Madrid)

Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


