12 Pathology of Lung Transplantation
Lung transplantation may offer longer survival and improved quality of life to patients with end-stage lung disease. Common indications for single lung, bilateral (double) lung, and heart-lung transplantation are listed in Table 12-1. Bilateral lung transplantation is the norm for cystic fibrosis, but of interest, the proportion of bilateral lung transplantation procedures has been rising for other major indications as well.1 Living donor single lobe transplantation may be a viable alternative to cadaveric lung transplantation for selected patients.2 Benchmark survival rates for adult lung transplant recipients are 88% at 3 months, 78% at 1 year, 63% at 3 years, 51% at 5 years, and 28% at 10 years after transplantation.1
Table 12-1 Most Common Indications for Lung Transplant Procedures
| Transplant Procedure | Most Common Indications |
|---|---|
| Adult single lung | Chronic obstructive pulmonary disease Idiopathic pulmonary fibrosis α1-Anti-trypsin deficiency emphysema |
| Adult bilateral/double lung | Cystic fibrosis Chronic obstructive pulmonary disease Idiopathic pulmonary fibrosis α1-Anti-trypsin deficiency emphysema Idiopathic pulmonary arterial hypertension |
| Adult heart-lung | Congenital heart disease Idiopathic pulmonary arterial hypertension Cystic fibrosis |
| Pediatric lung | Cystic fibrosis “Primary pulmonary hypertension” Congenital heart disease “Interstitial pneumonitis” Surfactant protein B deficiency |
Unfortunately, the number of patients who can benefit from lung transplantation is limited by the availability of donor organs. Historically, waiting time was the main determinant of donor lung allocation in the United States. In 2005, a lung allocation score (LAS) was implemented, dramatically changing the way donor lungs are distributed.3 Under the new system, priority for transplantation is determined by medical urgency and expected outcome. Early evaluations of the new system indicate that the waiting time and waitlist mortality rate are decreased, the number of transplants is increased, and the post-transplantation survival is unchanged.4
Complications of lung transplantation may be related to (1) the operation itself (primary graft dysfunction, anastomotic complications), (2) the host’s immunologic response to the allograft (rejection), and (3) the immunosuppressive therapy used to prevent rejection (infection, post-transplantation lymphoproliferative disorders [PTLDs]). Other complications, such as organizing pneumonia and recurrence of the original disease, may also occur. To aid the differential diagnosis, post-transplant time intervals can be divided arbitrarily into immediate (within 4 days), early (4 days to 1 month), and late (beyond 1 month) post-transplantation periods.5 Differential diagnostic possibilities for each of these periods are listed in Table 12-2.
Post-transplantation transbronchial biopsy may be performed for a specific clinical indication or for surveillance of acute rejection. The role of surveillance biopsy in lung transplant patients remains controversial.6,7 At least five pieces of well-expanded alveolated lung parenchyma are required for the assessment of acute rejection.8 The histopathologic findings most commonly encountered in a post-transplantation transbronchial biopsy include acute rejection, cytomegalovirus infection, airway-centered inflammation, pneumonia, bronchiolitis obliterans, harvest injury, invasive aspergillosis, and PTLDs.6,9
Operation-Related Complications
Primary Graft Dysfunction
Despite many advances in organ preservation, surgical technique, and perioperative care, primary graft dysfunction, also known as harvest injury, ischemia-reperfusion injury, early graft dysfunction, and reimplantation response, contributes significantly to both the morbidity and mortality for lung transplantation. Primary graft dysfunction affects an estimated 10% to 25% of pulmonary allografts and can range in clinical severity from transient decrease in oxygenation to complete graft failure.10 The International Society for Heart and Lung Transplantation (ISHLT) has proposed a definition and a grading scheme based on the chest film and Pao2/Fio2 ratio.11
Diagnosis
The diagnosis of primary graft dysfunction is based on the radiographic and Pao2/Fio2 criteria as well as on exclusion of clinically similar conditions such as acute antibody-mediated rejection, venous anastomotic obstruction, cardiogenic pulmonary edema, and pneumonia.11 In selected cases, a lung biopsy may be helpful.12
Pathologic Findings
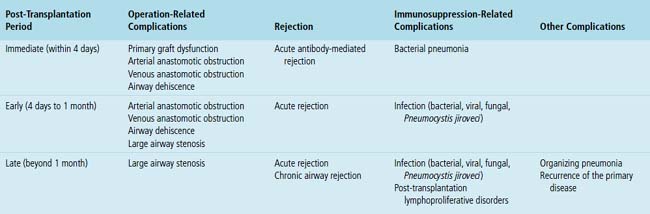
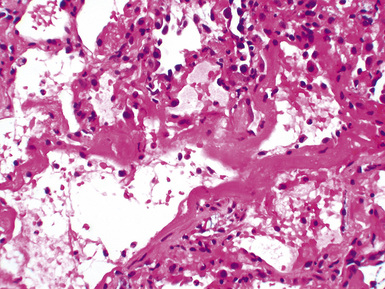
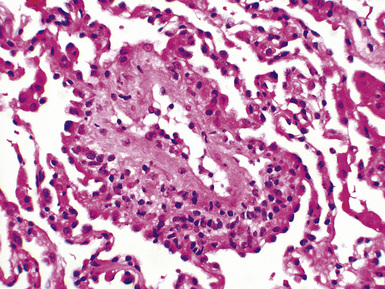
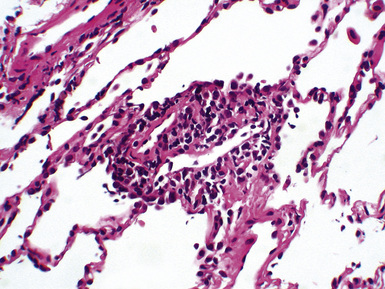
Mild cases may show alveolar and interstitial edema with scattered neutrophils.13 The histologic correlate of severe primary graft dysfunction is diffuse alveolar damage.12 The acute phase of diffuse alveolar damage is characterized by hyaline membranes, interstitial edema, occasional fibrin thrombi, and scattered neutrophils in the alveolar septa (Fig. 12-1). In the organizing phase, hyaline membranes are incorporated into the alveolar septa, which become thickened by fibroblast-rich connective tissue (Fig. 12-2).
Histologic Differential Diagnosis
Diffuse alveolar damage is a nonspecific histologic pattern that can be elicited by various insults in the post-transplantation setting (Box 12-1). Immunofluorescent studies are helpful in separating primary graft dysfunction from acute antibody-mediated rejection. Acute antibody-mediated rejection is characterized by alveolar septal deposits of IgG and complement (particularly C4d), which are absent in primary graft dysfunction. Acute rejection is not a major concern during the immediate post-transplantation period. Any infection can manifest as diffuse alveolar damage in an immunocompromised patient; therefore, it is always prudent to perform special stains to rule out acid-fast bacilli and fungal organisms.
Treatment, Prognosis, and Prevention
The treatment is supportive and may include mechanical ventilation. A retrospective analysis by Christie and associates showed that in patients with and those without primary graft dysfunction, 30-day mortality rates are 42.1% and 6.1%, respectively.14 Primary graft dysfunction is also associated with an increased risk of obliterative bronchiolitis.15 For prevention of primary graft dysfunction, research studies have focused on improving lung preservation techniques by optimizing the volume, temperature, pressure and components of preservation solutions, and inflation and ventilation parameters of the organs during transport.10 So far, these studies have had modest clinical impact.
Arterial Anastomotic Obstruction
The incidence of pulmonary arterial anastomotic obstruction after lung transplantation is relatively low.16 Causes include narrowed anastomosis, with or without thrombus formation resulting from suboptimal surgical anastomoses and excessive length of donor or recipient pulmonary artery with kinking or torsion of the anastomosis.16
Time Period
Arterial anastomotic obstruction usually occurs during the first week after transplantation.
Clinical Presentation
Signs and symptoms include dyspnea, hypoxemia, and elevated pulmonary arterial pressure.
Venous Anastomotic Obstruction
Minor abnormalities of the pulmonary venous anastomosis are relatively common complications of lung transplantation.17 Occlusive thrombus formation is relatively rare but may have catastrophic consequences, including allograft failure and stroke.
Time Period
Venous anastomotic obstruction usually presents in the immediate post-transplantation period but has been reported to occur as late as the eighth postoperative day.16
Clinical Presentation
Pulmonary venous obstruction after lung transplantation should be suspected in every case of persistent pulmonary edema in the first postoperative days, often associated with a frothy blood-stained secretion from the endotracheal tube.18
Diagnosis
Transesophageal echocardiography with color-flow Doppler imaging is virtually diagnostic, demonstrating a marked reduction of the flow in the affected pulmonary vein.18
Airway Dehiscence
In the early years of lung transplantation, airway dehiscence due to ischemia of the donor bronchus was a major cause of morbidity and death. Improved surgical techniques, reduced immunosuppression, and better allograft preservation have reduced the incidence of airway complications.19 Currently, most centers report a 7% to 18% complication rate with a related mortality rate of 2% to 4%.19
Diagnosis
Ischemia and necrosis of the bronchus can be diagnosed by direct visualization with a bronchoscope.
Large Airway Stenosis
Large airway (bronchial) stenosis is the most common airway complication. The incidence is estimated to be between 1.6% and 32%.19 It is usually seen after necrosis or dehiscence or in healing or treated infections. “Telescoped” anastomosis is associated with a 7% incidence of airway stenosis.
Nonanastomotic large airway stenosis has also been described.20,21 The pathogenesis of this lesion is unclear, but it may represent a response to ischemic damage, alloreactive injury, or infection.
Time Period
Bronchial stenosis usually occurs a few months after the transplantation procedure but has been described as early as 8 days.22
Diagnosis
Bronchoscopic examination provides the diagnosis, with biopsies providing confirmatory histology.
Pathologic Findings
Common findings include prominent granulation tissue, fibrosis, and squamous metaplasia.
Rejection
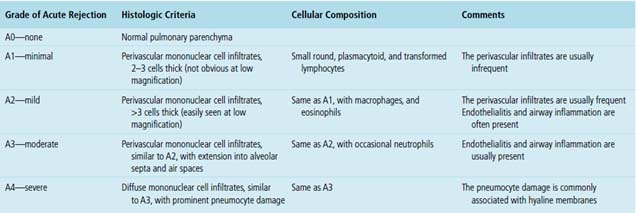
With the exception of monozygotic twins, donors and recipients are genetically different and express different histocompatibility antigens. As a result, allografts are rejected by the recipient’s immune system. Multiple immunologic processes are involved, creating a spectrum of rejection responses. A “working formulation for the classification of pulmonary allograft rejection” was introduced by the ISHLT in 1990.24 The working formulation was first revised in 1996.25 The currently accepted scheme for grading pulmonary allograft rejection was approved by the ISHLT board of directors in 2007 (Box 12-2).8 The differences between the 1996 and 2007 schemes are relatively minor and are related to airway inflammation and chronic airway rejection. Acute antibody-mediated rejection is a controversial subject and is not included in the 2007 working formulation. For the sake of completeness, however, it is discussed at the end of this section.
Box 12-2 2007 Revised Working Formulation for Classification and Grading of Pulmonary Allograft Rejection
Acute (Cellular) Rejection
Diagnosis
Clinical features may suggest acute rejection, but a transbronchial biopsy usually is required to confirm the diagnosis and rule out infection. If biopsy from multiple sites is technically impossible, lower lobe biopsies are preferred because they appear to be more informative.26
Pathologic Findings
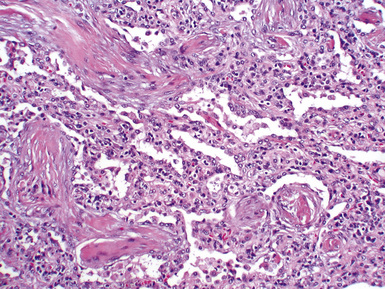
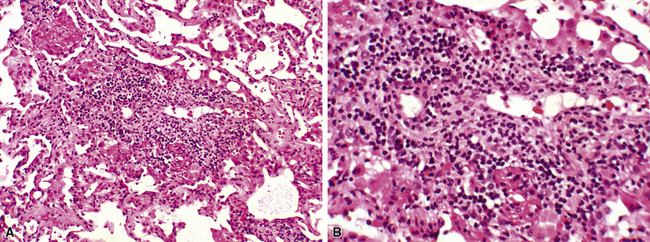
Acute rejection is graded according to the density and extent of the perivascular infiltrates and the presence or absence of secondary pneumocyte damage (Table 12-3). Rejection-type infiltrates usually involve more than one vessel, but a single perivascular infiltrate should be evaluated by the same criteria as for multiple infiltrates, as follows:
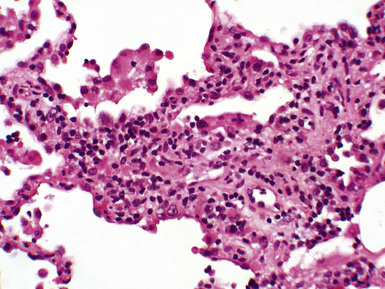
Figure 12-5 In moderate acute rejection (A3), the perivascular infiltrate extends into the alveolar septa.
Histologic Differential Diagnosis
Perivascular and interstitial mononuclear cell infiltrates are not specific for acute rejection.8 Differential diagnostic considerations include infections, especially cytomegalovirus pneumonia and Pneumocystis jiroveci pneumonia,27–29 and PTLDs.30 Some histologic features may favor infection over acute rejection (Table 12-4). Cultures and special stains may be helpful in the diagnosis of mycobacterial, fungal, and Pneumocystis jiroveci infections. Viral pneumonias can be confirmed by cultures as well as serologic, immunohistochemical, or molecular hybridization techniques.
Table 12-4 Histologic Features Favoring Infection over Acute Rejection
| Histologic Features | Infection Favored |
|---|---|
| Predominant alveolar septal infiltrates as compared with perivascular infiltrates | Any infection |
| Abundant neutrophils | Bacterial pneumonia, CMV pneumonia, or candidiasis |
| Abundant eosinophils | Fungal infection |
| Nuclear or cytoplasmic inclusions | Viral pneumonia |
| Multinucleation | Respiratory syncytial virus or parainfluenza virus pneumonia |
| Punctate zones of necrosis | Herpes simplex virus, varicella-zoster virus, or CMV pneumonia |
| Granulomatous inflammation | Mycobacterial, fungal, or Pneumocystis jiroveci infection |
| Frothy intra-alveolar exudates | Pneumocystis jiroveci pneumonia |
CMV, cytomegalovirus.
In some cases, histologic features of acute rejection and infection coexist. In these cases, the pathologist should attempt to decide which is dominant and guide the clinician by favoring one over the other. Follow-up biopsy after appropriate antimicrobial therapy is also recommended so that any acute rejection component can be reassessed.8 The differential diagnosis between acute rejection and PTLDs is discussed later.
Treatment and Prognosis
The treatment of acute rejection typically consists of bolus therapy with intravenous steroids, which may be supplemented by temporary increases in the maintenance immunosuppression regimen. In at least 80% of the cases, acute rejection is successfully treated. However, 15% to 20% of acute rejection episodes persist or recur, presenting a particularly difficult management problem for the clinician. When this occurs, intensified immunosuppression with one or more agents is usually attempted. However, it has been shown that patients with persistent, recurrent, or late (occurring at least 3 months after transplantation) acute rejection are at increased risk for developing chronic airway rejection.31 Recent studies have indicated that an increased risk may exist even with minimal acute rejection.32,33
Airway Inflammation: Lymphocytic Bronchiolitis
The 2007 working formulation has collapsed the four previous B grades into two (grade 1R—low grade and grade 2R—high grade) and has retained B0 (no airway inflammation) and BX (ungradable). Another change from the previous working formulation is that the B grade designation applies only to small airways (bronchioles). Airway inflammation may be a harbinger of chronic airway rejection.34,35
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree