The aim of this study was to test the hypothesis that ear oximetry immediately after the release of a sustained Valsalva maneuver accurately detects patent foramen ovale (PFO). One hundred sixty-five scuba divers underwent transesophageal echocardiography (TEE; reference method) for PFO assessment. Ear oximetry of the right earlobe was performed in a different room within a time frame of 2 hours before or after TEE. The subject and the oximetry operator were unaware of the results of TEE. Oxygen saturation (SO 2 ) measurements were obtained at baseline and during the release phase of 4 Valsalva maneuvers within 10 minutes, and the average SO 2 change (SO 2 at baseline minus SO 2 at Valsalva release) was determined as the primary study end point. One hundred seventeen divers had no PFO, and 48 (29%) had PFO by TEE (mean age 39 ± 8 years). The average SO 2 change was 0.79 ± 1.13% (i.e., a slight absolute SO 2 decrease in response to the Valsalva maneuver) in the group without PFO and 1.67 ± 1.19% in the PFO group (p <0.0001). Using receiver-operating characteristic curve analysis, a PFO as defined by TEE could be detected at a threshold of a Valsalva-induced decrease in SO 2 of ≥0.825 percentage points in comparison to baseline (sensitivity 0.756, specificity 0.706, area under the receiver-operating characteristic curve 0.763, p <0.0001, negative predictive value 0.882). In conclusion, the entirely noninvasive method of ear oximetry in response to repetitive Valsalva maneuvers is accurate and useful as a screening method for the detection of a PFO, as shown in this study of divers.
Even without scientific evidence, decompression illness (DCI) events in divers related to the presence of patent foramen ovale (PFO) are prevented by refraining from diving. PFO detection by a simple noninvasive screening method would be useful to limit discomfort and the cost of the examination. More than 50 years ago, Lüthy et al described the behavior of arterial oxygen saturation (SO 2 ) as obtained from the earlobe in patients with various congenital heart diseases, including those with right-to-left shunts. During release of a Valsalva maneuver, an SO 2 decrease can be observed in patients with temporary right-to-left shunts in atrial septal defects. In this context, in the present study we tested the hypothesis that ear oximetry immediately after the release of a Valsalva maneuver accurately detects PFO as proved by transesophageal echocardiography (TEE).
Methods
A total of 165 divers were recruited through several Swiss amateur diving clubs. All divers underwent TEE and ear oximetry in 2 separate rooms within a time frame of 2 hours. The 2 examinations were carried out by 2 different operators unaware of the results of the other test. The study subjects were not informed about the test results until after the completion of TEE and ear oximetry. The sequence of TEE and ear oximetry was not randomized but selected according to logistic aspects during the study exam sessions. As the reference exam, all study subjects underwent contrast TEE for the diagnosis and study group assignment of absent or present PFO ( Figure 1 ). PFO was absent in 117 divers and present in 48 (29%).
The study was approved by the ethics committee of the canton of Bern, Switzerland, and the study subjects gave written informed consent to participate in the study.
Before the study subjects were examined using TEE and ear oximetry, their medical histories, including alcohol intake, smoking habit, and medication use, were assessed. A detailed diving history was obtained. The absence or presence of DCI events were recorded. A DCI event was classified as minor or major. Minor DCI events were scored (0 to 3) according to the frequency of their occurrence (never, rarely, every fourth to third dive, or every second dive or more often). Minor DCI symptoms included bends, cutaneous erythema, extreme fatigue, headache, dizziness, paresthesia, and tinnitus. Major DCI events were defined by ≥1 of the following symptoms: limb weakness, cutaneous sensory level, impaired bladder or bowel control, paresis or paraplegia, blurred vision, dysarthria, amnesia for the event, hemiplegia, or loss of consciousness after a dive.
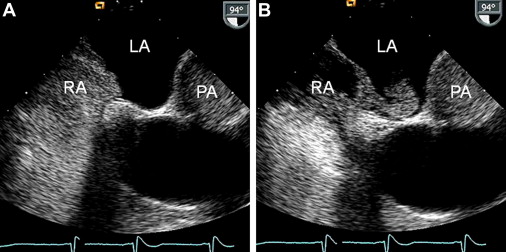
Before intubation of the TEE probe, the epipharynx was anesthetized using lidocaine hydrochloride 10% spray. A 3-lead electrocardiogram and blood pressure were monitored during TEE. No sedative medication was used in any of the study subjects. TEE was performed in the left lateral supine position of using a Siemens Acuson Sequoia C256 Doppler echocardiographic system (Siemens Medical Solutions USA Inc., Mountain View, California) with a multiplane 3.5- to 7-MHz probe. Documentation of PFO status occurred in the transversal, short-axis, and longitudinal ( Figure 1 ) image planes. The echocardiographic contrast medium for the detection of a right-to-left atrial shunt consisted of an ad hoc sonicated mixture of 0.5 ml air and 4.5 ml of a gelatin containing plasma expander. Echocardiographic contrast tests were performed in the 2 mentioned image planes by injection of 5 ml contrast into the right antecubital vein. Using a Valsalva maneuver (strain phase starting simultaneously with the contrast bolus injection), a leftward deviation of the interatrial septum in the fossa ovalis region ( Figure 1 ) was observed immediately after release of the Valsalva strain phase (lasting 5 to 10 seconds); this was observed in all subjects and was taken as a sign of a successful Valsalva maneuver. The diagnosis of PFO required the crossing of bubbles from the right to the left atrium ( Figure 1 ) within 4 heartbeats after the release of the Valsalva strain phase. The degree of PFO was qualitatively characterized by a score of 0 to 3, with a score of 1 representing the crossover of a few single bubbles and a score of 3 representing the shunt of an entire cloud of bubbles ( Figure 1 ).
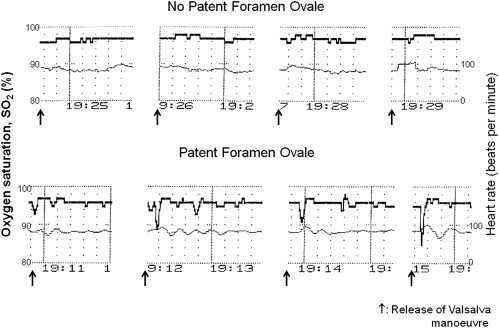
Pulse oximetry was performed using a 2-wavelength sensor (Nellcor; Covidien, Mansfield, Massachusetts) placed at the right earlobe. Arterial SO 2 (i.e., the percentage of hemoglobin bound with oxygen molecules) was determined 6 times at baseline with the subject in the supine back position. The first baseline measurement was taken after 2 minutes in the supine back position; the other baseline measurements were taken every 2 minutes thereafter. Subsequently, 4 Valsalva maneuvers were performed within 10 minutes, and during each, arterial SO 2 was obtained immediately after release of the strain phase of the Valsalva maneuver ( Figure 2 ). If several SO 2 decreases were present after the Valsalva maneuver release, the largest was taken for data analysis. All baseline and respectively all Valsalva SO 2 measurements were averaged, and the difference between average baseline values and average Valsalva maneuver release values represented the primary study end point. Secondary study end points consisted of averaged SO 2 baseline values alone, averaged Valsalva maneuver release values alone, or the SO 2 change after the first Valsalva maneuver release. Subjects were asked to perform the Valsalva maneuver according to the spoken instructions of the investigator (identical instruction during TEE): he or she should fully breathe in and fully breathe out and start the Valsalva strain phase as forcefully as possible immediately thereafter by closing the glottis and strain the abdominal musculature; after 7 to 10 seconds, the subject was asked to release the strain phase without immediately breathing in.
The distribution of the data for normality was tested using the Kolmogorov-Smirnov test. Characteristics between the groups were analyzed using unpaired Student’s t tests for continuous data and chi-square tests for categorical data. Analysis of the primary study end point according to PFO degree (score 0 to 3) was performed by analysis of variance followed by a Scheffé post hoc test. To determine interobserver agreement, 2 observers blinded to each other and to the results of TEE independently assessed the SO 2 recordings. Kappa statistics were used to measure the amount of agreement. The best cutoff and accuracy of arterial SO 2 measurements by ear oximetry for the detection of PFO as defined by TEE was determined using receiver-operating characteristic (ROC) curve analysis.
Results
The 117 divers without and 48 divers with PFO did not differ with regard to medical data ( Table 1 ). Data on PFO grades, diving histories, and DCI events are listed in Table 2 .
| Variable | No PFO (n = 117) | PFO (n = 48) | p Value |
|---|---|---|---|
| Age at study inclusion (yrs), mean ± SD | 38 ± 8 | 41 ± 8 | 0.13 |
| Men | 90 (77%) | 44 (92%) | 0.0290 |
| Medical data | |||
| Alcohol use daily | 11 (9%) | 4 (8%) | 0.99 |
| Smoking | 28 (24%) | 17 (35%) | 0.18 |
| Diabetes mellitus | 2 (2%) | 0 | 0.99 |
| Systemic hypertension | 8 (8%) | 3 (6%) | 0.99 |
| Heart disease | 0 | 3 (6%) | 0.24 |
| Respiratory disease | 3 (3%) | 1 (2%) | 0.99 |
| Migraine or chronic headache | 7 (6%) | 6 (6%) | 0.20 |
| Past surgical operations | 61 (52%) | 27 (56%) | 0.20 |
| Medication use daily | 20 (17%) | 5 (10%) | 0.34 |
| Variable | No PFO (n = 117) | PFO (n = 48) | p Value |
|---|---|---|---|
| Degree of PFO | |||
| 0 | 117 | 0 | |
| 1 | — | 10 | |
| 2 | — | 20 | |
| 3 | — | 18 | |
| Yrs of diving | 9.9 ± 6.6 | 12.0 ± 8.1 | 0.07 |
| Cumulative number of dives | 660 ± 747 | 1045 ± 1237 | 0.0154 |
| Number of dives per yr | 69 ± 42 | 79 ± 52 | 0.23 |
| Diving depth (m) | 29 ± 9 | 30 ± 9 | 0.83 |
| Cumulative number of dives >40 m | 100 ± 224 | 215 ± 442 | 0.0305 |
| Breathing gas: compressed air | 90 (77%) | 26 (54%) | 0.0066 |
| DCI events | |||
| Minor DCI score (score 0–28) | 2.7 ± 1.9 | 3.2 ± 2.1 | 0.12 |
| Major DCI | 13 (11%) | 15 (31%) | 0.0029 |
| Major DCI/10° dives | 1.8 ± 6.3 | 9.8 ± 39.4 | 0.0332 |
| Major DCI >24 h | 3 (3%) | 7 (15%) | 0.0070 |
| Major DCI with stay in decompression chamber | 3 (3%) | 9 (19%) | 0.0009 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


